Computational modelling explains why blues and greens are brightest colors in nature

Researchers have shown why intense, pure red colors in nature are mainly produced by pigments, instead of the structural color that produces bright blue and green hues.
The researchers, from the University of Cambridge, used a numerical experiment to determine the limits of matt structural color—a phenomenon which is responsible for some of the most intense colors in nature—and found that it extends only as far as blue and green in the visible spectrum. The results, published in PNAS, could be useful in the development of non-toxic paints or coatings with intense color that never fades.
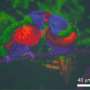
Structural color, which is seen in some bird feathers, butterfly wings or insects, is not caused by pigments or dyes, but internal structure alone. The appearance of the color, whether matt or iridescent, will depending on how the structures are arranged at the nanoscale.
Ordered, or crystalline, structures result in iridescent colors, which change when viewed from different angles. Disordered, or correlated, structures result in angle-independent matt colors, which look the same from any viewing angle. Since structural color does not fade, these angle-independent matt colors would be highly useful for applications such as paints or coatings, where metallic effects are not wanted.
"In addition to their intensity and resistance to fading, a matt paint which uses structural color would also be far more environmentally-friendly, as toxic dyes and pigments would not be needed," said first author Gianni Jacucci from Cambridge's Department of Chemistry. "However, we first need to understand what the limitations are for recreating these types of colors before any commercial applications are possible."
"Most of the examples of structural color in nature are iridescent—so far, examples of naturally-occurring matt structural color only exist in blue or green hues," said co-author Lukas Schertel. "When we've tried to artificially recreate matt structural color for reds or oranges, we end up with a poor-quality result, both in terms of saturation and color purity."
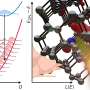
The researchers, who are based in the lab of Dr. Silvia Vignolini, used numerical modeling to determine the limitations of creating saturated, pure and matt red structural color.
The researchers modeled the optical response and color appearance of nanostructures, as found in the natural world. They found that saturated, matt structural colors cannot be recreated in the red region of the visible spectrum, which might explain the absence of these hues in natural systems.
"Because of the complex interplay between single scattering and multiple scattering, and contributions from correlated scattering, we found that in addition to red, yellow and orange can also hardly be reached," said Vignolini.
Despite the apparent limitations of structural color, the researchers say these can be overcome by using other kind of nanostructures, such as network structures or multi-layered hierarchical structures, although these systems are not fully understood yet.
More information: Gianni Jacucci et al, The limitations of extending nature's color palette in correlated, disordered systems, Proceedings of the National Academy of Sciences (2020). DOI: 10.1073/pnas.2010486117
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Cambridge