A new design for flow batteries

In 2020, China plans to launch the largest battery complex in the world with a capacity of 800 MW*h (approximately this amount of energy per year is consumed by a household with 200 apartments). This complex is based not on the usual lithium-ion or lead-acid batteries, but on the redox flow battery where the electricity is stored in the form of chemical energy of solutions—electrolytes. Battery consists of two tanks in which electrolytes are stored and membrane-electrode assembly (MEA)—solutions are supplied to MEA by pumps where they undergo electrochemical reactions which provide charging and discharging of the battery.
Due to this setup, redox flow batteries, unlike many other energy storage devices, enable independent scaling of power and capacity of the battery, which are determined by the size of MEA and electrolyte volume, respectively. In addition, redox flow batteries exhibit minimal self-discharge over extended periods and their electrolytes do not degrade even after tens of thousands of operating cycles, making them promising candidates for storing large amounts of energy in the smart power grids. For example, they can store excess electricity generated by photovoltaic solar cells during daylight and generate back-up electricity at night or in cloudy weather.
"Flow batteries are being actively integrated into the power grids of China, Germany and other countries, on one hand, and on the other hand, are continued to be developed and refined in laboratories," comments one of the authors of the work, researcher at the NTI Competence Center at IPCP RAS, Dmitry Konev. "We have proposed a completely new design of MEA, which will facilitate the research process and greatly reduce entrance threshold for new research groups into this area. In the future, this will help to achieve significant progress and will bring distributed energy resources from niche positioning to very high level of commercialization, including in Russia."
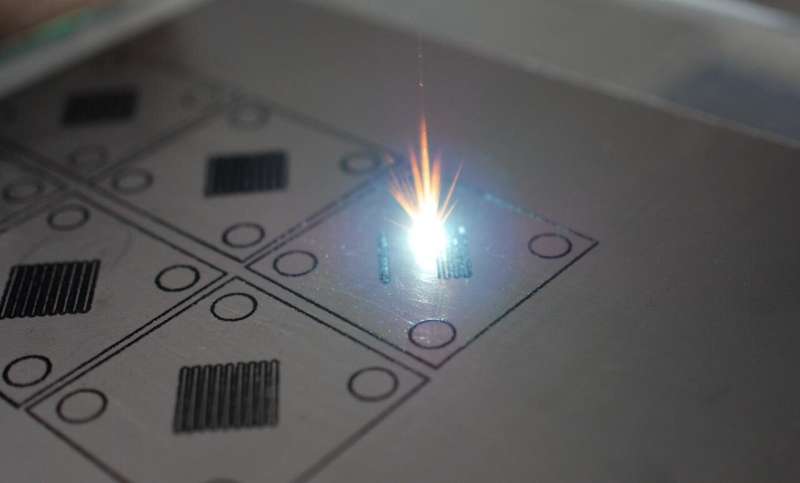
Sandwich with laser filling
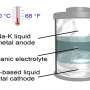
The MEA is the heart of the flow battery. It looks like a sandwich of different sheets materials, divided into two symmetrical parts supplied with its own electrolyte. When the battery is connected to a power supply, the one electrolyte is oxidized, while another is reducing and so the battery is charged. After that, the power source can be disconnected and replaced with an energy consumer—the electrolytes will undergo reverse processes and the battery will begin to discharge.
An important part of the MEA is the flow field plates, sandwich layers through which the electrolyte is pumped to the electrodes where the electrolytes are oxidized or reduced. The performance of the battery, i.e. power and efficiency, depend strongly on how well the flow fields are organized. Therefore, researchers often select different types of fields to optimize battery performance, but this is a very labor-intensive task: flow fields are milled in dense graphite plates, which is a time-consuming procedure. Russian researchers have proposed a different approach.
"We form flow fields by using several thin layers of flexible graphite materials: the necessary patterns in them are cutted by a laser and then these layers are superimposed on each other to get the required field," says the first author of the work, Roman Pichugov, a researcher at Mendeleev Univeristy. "The procedure to create flow fields takes only a few minutes, which is much less than traditional milling of graphite. Plus, cheaper materials are used, and as a result, there is more scope for variation and selection of flow fields."
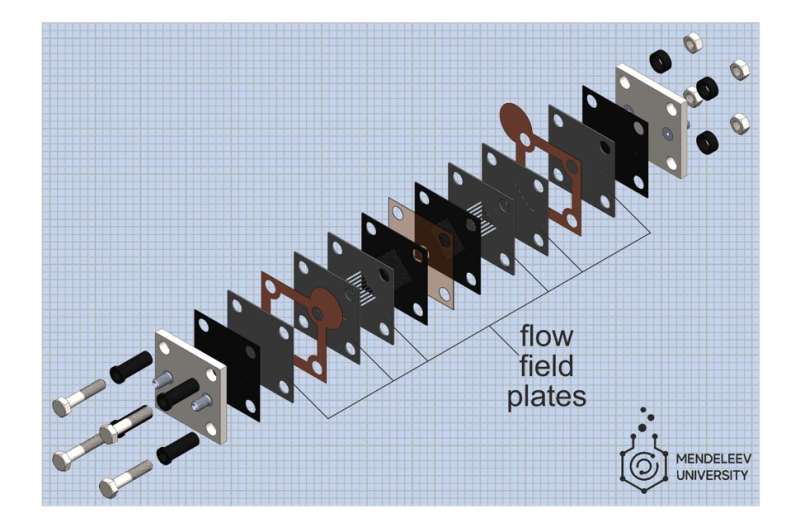
From cell to stack
Flow batteries can operate with different types of electrolytes. The most common (including those that are installed in China and are being introduced in other countries) utilize vanadium electrolytes, namely solutions of vanadium salts. This is the electrolyte on which Russian scientists tested their cell design. They sorted out various types of flow fields, varied the electrolyte flow rate and obtained results that on a qualitative level coincide with the best world studies and on a quantitative level even slightly surpass them: the power of tested MEA slightly exceeded the power of similar cells on graphite.
Thus, the new design of MEA is greatly simplifying laboratory tests and in the future can be used in real energy storage systems for distributed power grids. Russian scientists in collaboration with InEnergy LLC are developing and testing a vanadium flow battery composed of 10 such cells with a total power of 20 watts. The construction of the cell itself and the stack of 10 cells are protected by patents, the last of which belongs to the ADARM company, created by the employees of MUCTR. In addition, scientists are developing other types of flow batteries utilizing different electrolytes on the basis of the proposed design of MEA.
More information: Roman Pichugov et al, Electrolyte Flow Field Variation: A Cell for Testing and Optimization of Membrane Electrode Assembly for Vanadium Redox Flow Batteries, ChemPlusChem (2020). DOI: 10.1002/cplu.202000519
Provided by Mendeleev University