May 26, 2020 feature
Electron microscopy of nanoparticle superlattice formation at a solid-liquid interface in non-polar liquids
Nanoparticle superlattice films that form at the solid-liquid interface are important for mesoscale materials but are challenging to analyze on the onset of formation at a solid-liquid interface. In a new report on Science Advances, E. Cepeda-Perez and a research team in materials, physics and chemistry in Germany studied the early stages of nanoparticle assembly at solid-liquid interfaces using liquid-phase electron microscopy. They observed oleylamine-stabilized gold nanoparticles to spontaneously form thin layers on a silicon nitride membrane window of the liquid enclosure. In the first monolayer, the assembly maintained dense packings of hexagonal symmetry independent of the nonpolar solvent type. The second layer displayed geometries ranging from dense packing in a hexagonal honeycomb structure to quasi-crystalline particle arrangements—based on the dielectric constant of the liquid. The complex structures made of weaker interactions remained preserved, while the surface remained immersed in liquid. By fine-tuning the properties of materials involved in nanoparticle superlattice formation, Cepeda-Perez et al. controlled the three-dimensional (3-D) geometry of a superlattice, including quasi-crystals (a new state of matter).
Nanoparticles that are densely packed into two or three dimensions can form regular arrays of nanoparticle superlattices. For example, semiconductor particle superlattices can act as "meta" semiconductors when doped with particles to form new mesoscale materials, while plasmonic particles in dense superlattices can couple to form collective modes with angle-dependent and tunable wavelength responses. Large electric fields can occur between such particles for surface-enhanced Raman spectroscopy. Superlattices can be developed at liquid-liquid, gas-liquid and solid-liquid interfaces, where static and dynamic interactions between particle-substrate, particle-particle and particle-liquid interactions can dictate the structure of superlattices. However, it remains difficult to predict such structures in advance. For example, simulating the assembly of superlattices at multiple stages is not yet possible, with very little in-lab real-space data available for modeling. It is therefore challenging to gather experimental insights on fundamental mechanisms of superlattice formation.
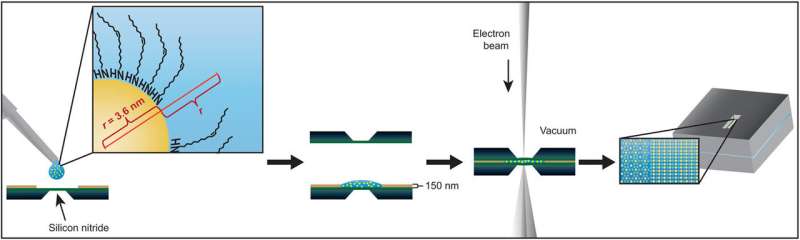
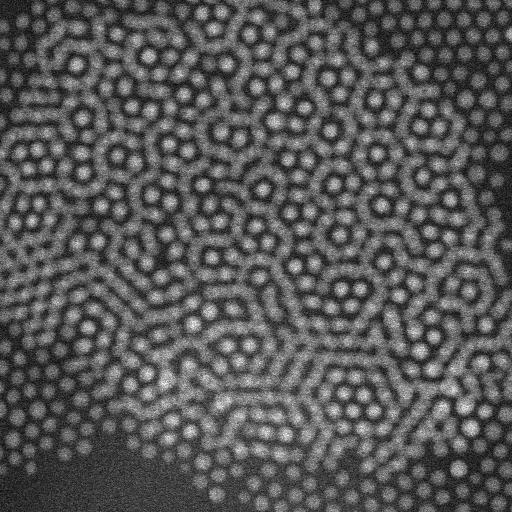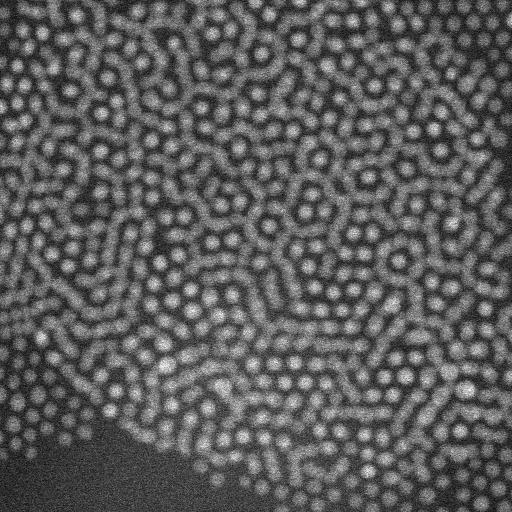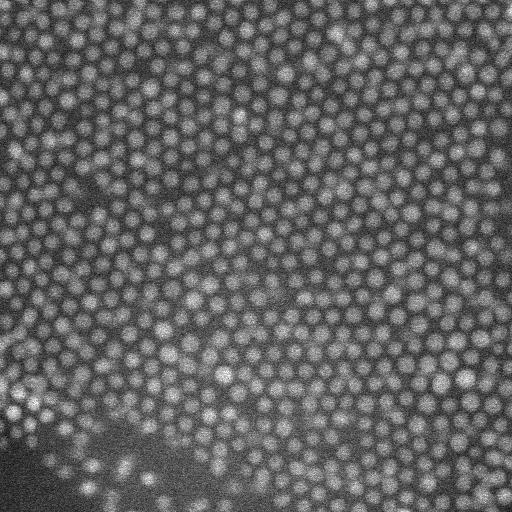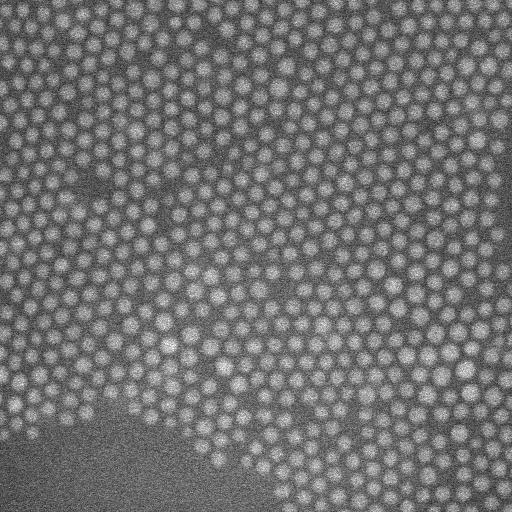
In this work, Cepeda-Perez et al. directly imaged superlattices of gold nanoparticles (AuNPs) suspended in different nonpolar solvents using liquid-phase electron microscopy (LP-EM). The AuNPs in the first layer above a surface, strongly interacted with the substrate and each other. As a superstructure assembled layer-by-layer, the surface interactions diminished, and the patterns encountered weaker forces between nanoparticles mediated by the properties of the surrounding liquid. The team studied transitions from particle-surface interactions to weaker interparticle interactions by examining patterns of a monolayer and a double layer, under different solvent conditions. To study the origin of superlattices they examined a single type of oleylamine-coated AuNPs assembled on a silicon nitride (SiN) coated membrane. To understand interactions between AuNPs and the substrate, the scientists changed the solvent and used scanning transmission electron microscopy (STEM) to obtain the highest spatial resolution possible with the lowest possible electron flux.
The team subjected the SiN (silicon nitride) membranes to an oxidizing plasma to induce surface polarity directly before loading the sample. Then using liquid-phase electron microscopy they observed the gold nanoparticles (AuNPs) located at the solid-liquid interface. They tested four different nonpolar liquids with different dielectric constants to vary the range of interactions. Particle-substrate interactions attracted the nanoparticles from the liquid to the SiN surface to form a thin film. The strong and weak interactions caused dense packings that were only weakly affected by the choice of solvent. The overall densities were highest for the linear-chain solvents.
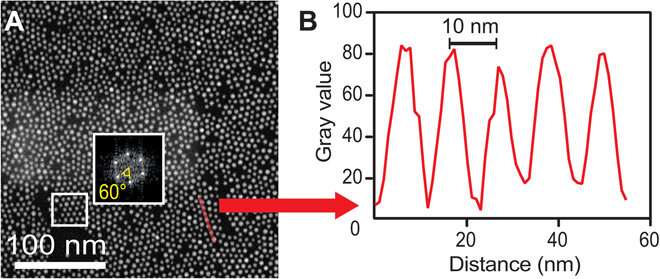
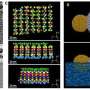
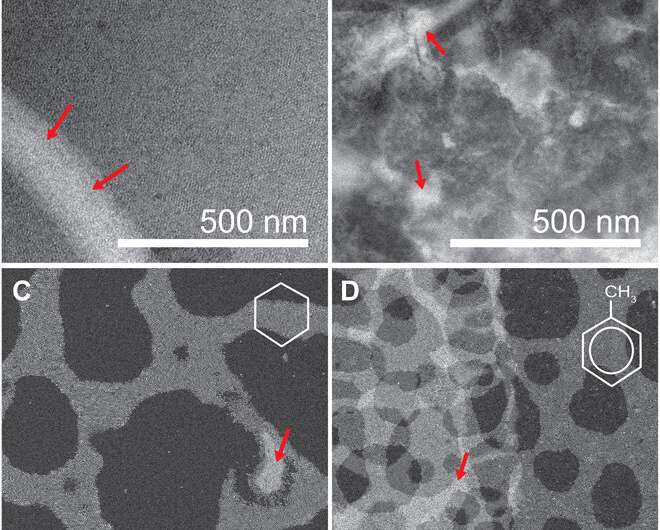
When imaging the AuNP layer in hexadecane solvent, the team observed brighter and fainter spots to indicate the presence of AuNPs on top of a monolayer—alongside three discrete levels from the signal of the image. The observed patterns remained stable even after they recorded images of the sample area in a time-lapse. Comparatively, cyclohexane non-polar organic solvents led to a dense, hexagonal top layer and the team noted structures with a hexagonal base and rhombitrihexagonal (characterized by one triangle, two parallelograms, and one hexagon on each vertex) tiling. The samples also showed patches of quasi-crystalline nature. Compared to the first layer of particles, the team observed weaker bonding of particles in the second layer, they credited reduced particle packing density to the decay of particle-substrate interaction potential.
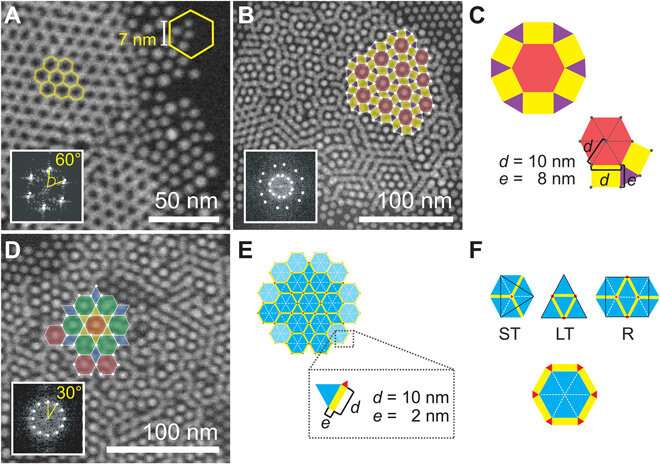
In this way, E. Cepeda-Perez and colleagues showed that solvents with high dielectric constants reduced the packing densities and allowed the formation of quasi-crystalline structures. The results were consistent with the hypothesis of the study on substrate-induced self-assembly and particle-substrate attraction. The LP-EM data explained the mechanisms behind the formation of self-assembled superlattices of gold nanoparticles at a solid-liquid interface. The outcomes led to quasi-crystalline particle arrangements, which depended on the strength of the dielectric constant of the liquid in the setup. Based on the results, the 3-D geometry of a superlattice including complex quasi-crystalline structures can be fine-tuned by manipulating the properties of the involved liquid, nanoparticles, and interface materials.
More information: E. Cepeda-Perez et al. Electron microscopy of nanoparticle superlattice formation at a solid-liquid interface in nonpolar liquids, Science Advances (2020). DOI: 10.1126/sciadv.aba1404
Angang Dong et al. Binary nanocrystal superlattice membranes self-assembled at the liquid–air interface, Nature (2010). DOI: 10.1038/nature09188
Eiji Abe et al. Quasicrystals as cluster aggregates, Nature Materials (2004). DOI: 10.1038/nmat1244
Journal information: Science Advances , Nature , Nature Materials
© 2020 Science X Network