Development of electrode material improving the efficiency of salinity gradient energy
Dr. Jeong Nam-Jo of the Korea Institute of Energy Research (KIER), Marine Energy Convergence and Integration Research Team developed synthesis technologies for an electrode material that can directly synthesize molybdenum disulfide thin films on the electrode current collector surface to contribute to improving the efficiency and economic feasibility of salt gradient power generation using reverse electrodialysis. The research result was published in Applied Surface Science, the world's leading authority in the field of surface science.
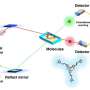
Reverse Electrodialysis (RED) is the principle of producing electricity using the electrical potential that occurs when ions between sea water and fresh water are separated and move through the ion exchange membrane in the stack. This technology is being actively pursued worldwide as a blue energy technology with high utilization and low variability in power production.
In reverse electrodialysis, the electrode catalyst serves to generate electricity by activating charge transport through an electrochemical reaction. However, since most methods use expensive materials such as platinum, it is necessary to develop a renewable technology to secure economic feasibility and capable of synthesizing inexpensive electrode materials at industrial scale.
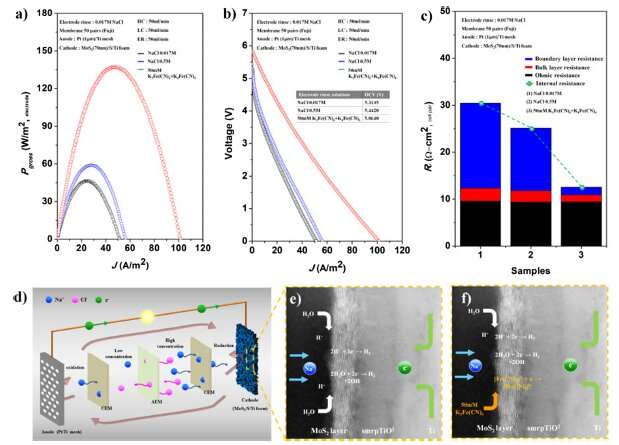
In order to overcome this, the research team has succeeded in developing the technology for directly synthesizing a highly active and also inexpensive molybdenum disulfide thin film as the main catalytic active sites on the surface of a current collector regardless of its substances (metal and carbon) and structural morphologies (one-dimensional, two-dimensional, or three-dimensional), which help to improve the electrochemical activity of the electrode catalyst.
In the conventional synthesis method, the complicated and more uneven structure of a current collector results in the non-uniform coating of electrode catalysts. This leads to lower and unstable catalytic activity, causing a decrease in performance as well as a large decrease in the used precursor. On the other hand, the research team succeeded in realizing a synthesis device capable of maintaining uniform concentration distribution on all surfaces of substrates with self-vaporization depending on the supply amount of precursor in the reactor. Therefore, it was possible to obtain a very uniform coating while minimizing the loss of used precursor, which results in the highest electrode performance.
In addition, since this technology is capable of large area synthesis, it can be applied to various research fields besides salinity gradient power generation, and is expected to make a great contribution to their commercialization.
Dr. Jeong said "With this synthesis technology, it is possible to replace electrode materials in the field of water treatment, which has a high level of dependence in import and is expensive, so it will contribute to the development of localizing materials and components in related fields. Also, this technology shows that KIER is a world-leading research group for salinity gradient technology."
More information: Namjo Jeong et al, Thickness-modulated and interface-engineered MoS2/TiO2 heterostructures as a highly active and inexpensive cathode for reverse electrodialysis, Applied Surface Science (2019). DOI: 10.1016/j.apsusc.2019.144323
Provided by National Research Council of Science & Technology