Single atom layer trap for lithium-ion migration
On April 14th, Prof. Ma Cheng from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) and his colleagues reported an important discovery regarding the mechanism of Li-ion migration in solid electrolytes for batteries, observing a new type of microscopic feature that can significantly influence ionic transport.
Solid electrolytes are the key components for safe, energy-dense, all-solid-state batteries. Before highly conductive solid electrolytes can be developed in a knowledge-based manner, the mechanism behind Li-ion migration must be thoroughly understood. In many materials, the success of this task lies in whether the "non-periodic features" can be well understood, because such features frequently cause an orders-of-magnitude change in ionic conductivity. At present, only two types of non-periodic features, grain boundaries and point defects, were considered in most studies.
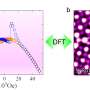
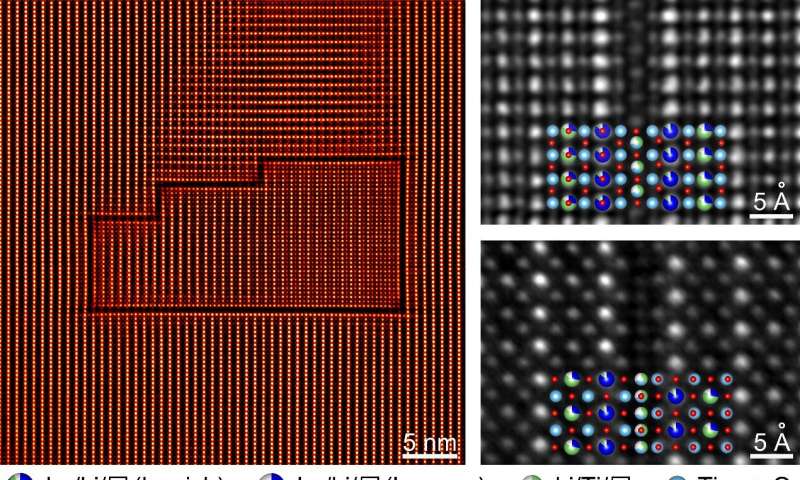
Ma's team discovered an additional type of non-periodic feature that profoundly affects ionic transport. Using aberration-corrected transmission electron microscopy, they spotted a large number of single-atom-layer defects in a prototype solid electrolyte Li0.33La0.56TiO3. In contrast to other well-known non-periodic features, the observed defect is essentially a single-atom-layer compound that emerges only on a limited number of atomic planes. Because of the symmetry of these planes, differently oriented defects almost always form closed loops.
"There are actually many such defect loops in the material, but it is very difficult to observe them," said the first author ZHU Feng, who is currently a Ph.D. student of USTC. "They are visible only along certain orientations. Additionally, due to their extreme thinness and the distraction from other coexisting microstructures, the presence of these defects is hardly noticed. This might explain why they have not been reported until now."
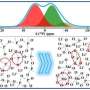
The observed defects were found to exhibit an atomic configuration that completely forbids Li-ion migration across the defect layer. As a result, when such defects form a closed loop, Li ions can neither enter nor exit the volume inside, and this part of material is thus excluded from the overall ionic transport. The volume isolated this way is as high as ~15%, which can lead to a one to two orders-of-magnitude reduction in ionic conductivity.
"The defect loop is acting as a Li-ion trap: it prevents the Li ions within the enclosed volume from escaping," said Prof. Ma Cheng from USTC, the lead author of the study. "As such, although the defects themselves are only one atom thin, they still can 'kill' very large volumes of the solid electrolyte, making them non-conductive."
The scientists coined the term "single-atom-layer trap" (SALT) to describe this unique feature. Its discovery reveals that non-periodic features other than grain boundaries and point defects may also greatly alter ionic transport, and that similar studies are urgently needed on other solid electrolytes.
More information: Feng Zhu et al, Single-atom-layer traps in a solid electrolyte for lithium batteries, Nature Communications (2020). DOI: 10.1038/s41467-020-15544-x
Journal information: Nature Communications
Provided by University of Science and Technology of China