February 6, 2020 report
Structure of human thyroglobulin identified
A team of researchers from the U.K., Slovenia and Germany has determined the structure of human thyroglobulin using cryo-electron microscopy. In their paper published in the journal Nature, the group describes their study of the protein and its structure. Nancy Carrasco with Vanderbilt University has published a News & Views piece outlining the work by the team in the same journal issue.
The thyroid gland is located in the front of the neck below the Adam's apple. It is responsible for creating hormones that regulate metabolism in almost all cells in the body—the same hormones also play an important role in the development of muscles, the skeleton and the central nervous system. The gland is made up of spherical follicles, each of which are fluid-filled and covered in a layer of follicle cells. Thyroglobulin is stored in the fluid, which is called colloid. Prior research has shown that thyroglobulin is synthesized in organelles inside the follicular cells. And though thyroglobulin has been known to be heavily involved in the synthesis of thyroid hormones, until now, its structure has not been fully understood.
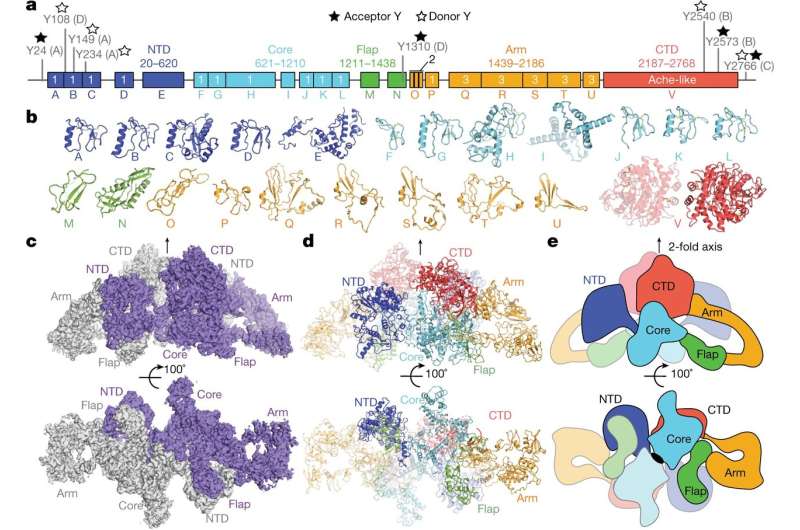
The work involved culturing specially engineered follicular cells to produce a large amount of pure thyroglobulin. The researchers studied the protein using cryo-electron microscopy. As the study progressed, the researchers used what was learned to build an atomic model of the protein. At its conclusion, the model showed the thyroglobulin structure to have five main regions: flap, core, carboxy-terminal domain, arm and amino-terminal domain. The model also showed the protein's tyrosine amino-acid residues, which are used to form hormones. And it showed that the two monomers were intertwined and that the amino-terminal domain in each monomer interacted with all five of the regions of the second monomer. They also found that the residues they had identified were the only proteins that were homogenic out of 67 in each monomer.
Carrasco points out that in addition to providing new details on the structure of thyroglobulin, the work should lead to a better understanding of the factors that can lead to hypothyroidism, a condition in which the thyroid gland is abnormally slow. People with the condition experience physical and mental retardation.
More information: Francesca Coscia et al. The structure of human thyroglobulin, Nature (2020). DOI: 10.1038/s41586-020-1995-4
Journal information: Nature
© 2020 Science X Network