Separation chemistry: A step toward greener metal recycling processes

Liquid-to-liquid extraction is the basic process used in hydrometallurgy for recycling metals and decontaminating solvents (to recover molecules that can be re-used or to decontaminate them). Until now, the "recipes" used in chemical processes have been based on operating feedback and theories that are only partly understood. No physical and chemical predictive models have been available to optimize experiments, particularly in the case of using several extractants together.
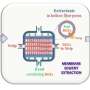
The teams at the Institute of Separative Chemistry in Marcoule (CEA/CNRS/ENSCM/Université de Montpellier) and their foreign partners have successfully identified and explained the mysterious synergy that operates between extractants, a phenomenon that has been known to exist since the 1960s but which has never been explained until now. The researchers call this an ienaics approach. It provides new insight into the physical and chemical interactions that occur beyond immediate neighbors in a solution. To this end, experiments and measurements that are ten times more precise than any published to date were carried out using the instrumented bench assembled at Marcoule and deployed at the SCARCE laboratory in Singapore (see second image below), to rigorously quantify, for the first time, the efficiency of the extractant molecules and the synergy between them.
At the same time, the CEA, in partnership with CNRS and the Universities of Regensburg and Montpellier, patented this new method, covering the combination of conventional extractants mixed with non-extractant molecules in the hydrotrope class, a family of chemicals that have not, to date, been used in recycling, but which present synergistic behavior that had not been identified until now.
Finally, another paper confirms the potential industrial uses of the ienaics approach, by applying it as a predictive model for fluid viscosity in nuclear hydrometallurgy, something that has been a major obstacle to ramping up recycling processes, in addition to the turbidity of degreasing detergents.
These early days for ienaics have focused on improving the processing of heavy and strategic metals by extraction. In particular, it has been used to reduce the quantities of fluid used and, therefore, the amount of effluent produced. All this promises a new boost for hydrometallurgy processes, for example, for recycling wind turbine magnets in France and other EU Member States. Within the next ten years or so, advances in the technology are expected to result in more efficient and ecologically acceptable extraction processes at biofuel refineries and in cheaper purification methods for molecules for use in the pharmaceutical industry.
More information: Asmae El Maangar et al. A microfluidic study of synergic liquid–liquid extraction of rare earth elements, Physical Chemistry Chemical Physics (2020). DOI: 10.1039/C9CP06569E
Maximilian Pleines et al. A minimal predictive model for better formulations of solvent phases with low viscosity, EPJ Nuclear Sciences & Technologies (2020). DOI: 10.1051/epjn/2019055
Mario Špadina et al. Synergistic Solvent Extraction Is Driven by Entropy, ACS Nano (2019). DOI: 10.1021/acsnano.9b07605
Journal information: Physical Chemistry Chemical Physics , ACS Nano
Provided by CEA