August 15, 2019 feature
Controlled hydraulic fracturing sculpts mammalian embryos into shape
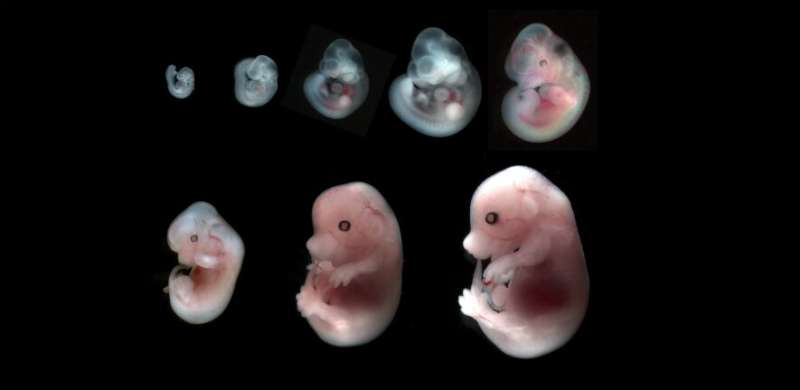
Hydraulic fracturing, or fracking, is a process most often associated with shale gas extraction, but a team of researchers from Institut Curie at the Sorbonne, and the Center for Interdisciplinary Research in Biology at the College de France have concluded that self-fracking is the mechanism that moves an embryo (here, a mouse) from a radially symmetric accumulation of cells to a bilaterally symmetric blastocyst.
Published in the journal Science in an article titled "Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst," and in an accompanying Perspectives piece titled "Embryonic self-fracking: Mammalian embryos use controlled hydraulic fracturing to sculpt their shape," the team's findings are laid out in clear steps. Many, we already know:
Before implantation, an embryo is a compact group of cells that divide more or less radially, maintaining a generally spherical shape. Then the cells become bilaterally symmetrical—a blastocyst—comprising a blastocoel filled with fluid, the inner embryonic cell mass that will eventually become a fetus, and a barrier called the trophectoderm that will eventually become the placenta. The fracking happens in the stage between these two steps; it's how the cells move their arrangement from radial to bilateral symmetry down the first axis.
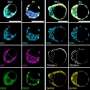
Using high-resolution live imaging, the team watched the process of symmetry breaking during the formation of a mouse blastocyst. As usual, the cells divided from a fertilized egg into two cells, then four, and so on, until the fifth round of cell division, when the team observed "the synchronous appearance of hundreds of bubbles at cell-cell junctions," each filled with pressurized water.
During the formation of these bubbles (microlumens), the cells' primary adhesion protein (E-cadherin) accumulates at the edges. Pressurized fluid from the blastocoel is injected, just like in shale gas fracking, between two cohesive cell membranes, breaking them apart and redistributing their E-cadherin to its new location at the edges of the newly formed microlumens.
After a period of profuse hydraulic fracturing, the coarsening phase began, with some bubbles growing larger, and then the larger microlumens conglomerating until they had formed one large lumen, which displaced the embryonic cell mass into one half of the blastocyst.
Although this is the first time the self-fracking process has been observed in live mouse blastocysts, it is not the first time researchers have observed the microlumen coarsening process. The study's authors report that "similar pressurized fluid bubbles that disrupt cell-cell and cell–extracellular matrix interfaces have been observed in vitro as a result of fluid pressure in the extracellular matrix, osmotic perturbations, or directed ionic transport."
More information: Julien G. Dumortier, et al. "Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst" Science 365 (6452), 465-468. 2019. DOI: 10.1126/science.aaw7709
Marino Arroyo and Xavier Trepat. "Perspectives: Embryonic self-fracking: Mammalian embryos use controlled hydraulic fracturing to sculpt their shape," Science, 2019. DOI: 10.1126/science.aay2860
L. Casares et al., Nat. Mater. 14, 343 (2015).
C. E. Morris, J. A. Wang, V. S. Markin, Biophys. J. 85, 223 (2003).
E. Latorre et al., Nature 563, 203 (2018).
© 2019 Science X Network