Next-generation medication: Where chemistry meets computation
A group of Japanese researchers mainly from Tokyo University of Agriculture and Technology (TUAT) and Hokkaido University drastically enhanced and sped up the way to producing skeletally diverse indole alkaloids, composed of the medicinally-relevant scaffolds. By leveraging computational and synthetic approaches, this group has successfully developed a concise and versatile synthetic process generating densely-functionalized multicyclic complex scaffolds, which would facilitate the development of both medicine and agrochemicals.
This synthetic approach employing zinc (II) reagent in place of Hg(II) or Gold-based reagents is also environmentally friendly as well as much cheaper than those that were used to-date. The research was published in Chemical Science on May 1st, 2019.
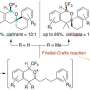
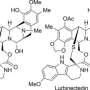
Plants and fungi that contain indole alkaloids have a long history of use in traditional medicine. In efforts to synthesize the complex alkaloids, chemists usually develop a customized synthetic strategy for efficient construction of the single targeted scaffold. In contrast, this synthetic approach allows concise and divergent synthesis of skeletally diverse alkaloidal scaffolds employing a common multipotent intermediate upon activation of alkyne moiety with zinc (II) reagent.
"We have successfully achieved programmable synthesis of the four distinct alkaloidal skeletons by implementing divergent annulations. The four kinds of annulation modes were demonstrated to be controlled in a programmable manner by appropriate choices of the substituents in the vicinity of the reaction centers, optimization of solvents and reaction conditions," says Hiroki Oguri, Ph.D., corresponding author and Professor at the Department of Applied Chemistry, Graduate School of Engineering, TUAT, Japan.
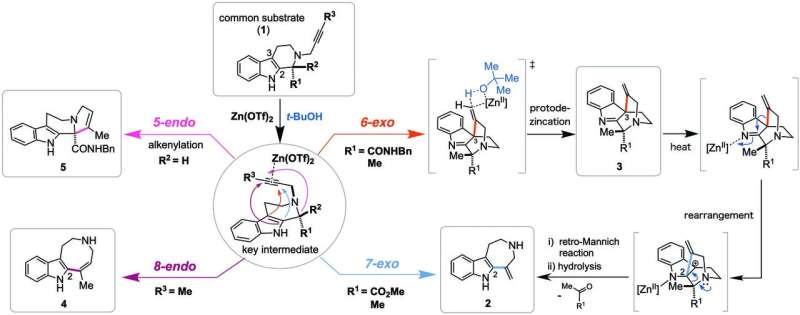
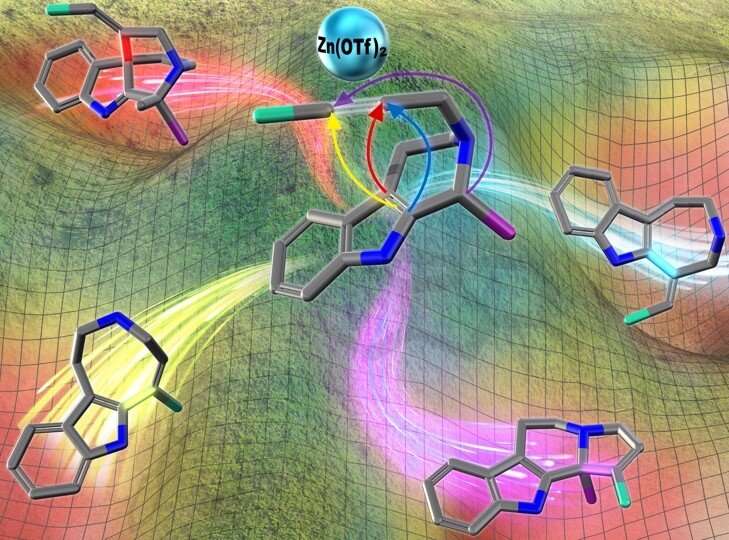
By integrating computational and experimental investigation methods, the researchers were able to not only gain insight into the reaction mechanism proposing unique transition states, but also to showcase a useful synthetic strategy for the concise and divergent access to the medicinally-relevant alkaloidal structures.
"These experimental findings underscore that Zn(OTf)2-mediated activation of alkynes provide relatively unexplored but versatile synthetic methodologies for the direct and flexible synthesis of alkaloidal scaffolds reminiscent of natural products," Oguri adds.
The researchers plan to implement their methods to create compounds other than just indole alkaloids, and they envision that their method could offer both rational and unexpected guidelines for designing other similar reactions. Integration of synthetic strategies for generating structural variations with a systematic computational approach for identifying unforeseen reaction pathways could provide a new route for advancing the combinatorial chemical synthesis of functional molecules. They also expect that this method will generate lead candidates for the development of next-generation pharmaceuticals and pesticides.
More information: Sadaiwa Yorimoto et al, Zn(OTf)2-mediated annulations of N-propargylated tetrahydrocarbolines: divergent synthesis of four distinct alkaloidal scaffolds, Chemical Science (2019). DOI: 10.1039/c9sc01507h
Journal information: Chemical Science
Provided by Tokyo University of Agriculture and Technology