July 2, 2019 feature
A counterintuitive case in which like charges attract
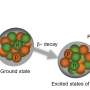
When it comes to electric charge, there is one overriding theme: opposites attract, and like charges repel. But in a new study, physicists have made the surprising discovery that two spherical like-charged metal nanoparticles with unequal charges can attract one another in a dilute electrolyte solution. The reason, in short, is that the more strongly charged nanoparticle polarizes the metal core of the weakly charged nanoparticle, which alters the interaction between the nanoparticles.
The researchers, Alexandre P. dos Santos and Yan Levin at the Federal University of Rio Grande do Sul in Brazil, have published a paper on the like-charge attraction in a recent issue of Physical Review Letters.
"Our paper elucidates a very counterintuitive behavior which was previously thought to be impossible," Levin told Phys.org.
This is not the first time that researchers have observed attraction between like-charged particles. As far back as 1980, research has shown that like-charged particles can attract one another when placed in an electrolyte solution containing multivalent counterions. A multivalent counterion is an ion that can lose or gain more than one electron to take on a charge such as ±2 or ±3, and the sign of the charge is opposite that of another ion. For example, the aluminum ion Al3+ is a multivalent counterion for the chloride ion Cl-, together forming aluminum chloride, AlCl3. When multivalent counterions are part of an electrolyte solution, their charges can fluctuate in a correlated way, causing like-charged particles in the solution to attract one another.
However, in the new demonstration, the electrolyte solution is 1:1, meaning it contains only monovalent counterions, i.e., ions that have only ±1 charge. As electrostatic correlations between ions in 1:1 solutions are negligible, it has generally been assumed that like-charged particles in these solutions always repel each other. In support of this assumption, in the new study the researchers demonstrated that like-charged metal slabs in a 1:1 electrolyte solution always repel each other.
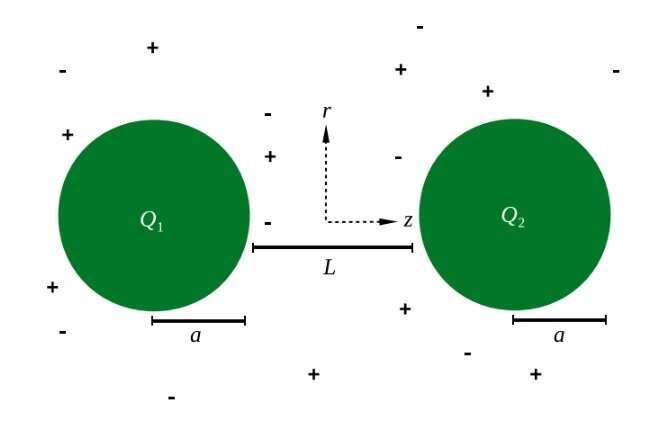
Until now, all of the previous studies in this area have only investigated situations in which the two like-charged particles have the same magnitude of charge. In the new study, the researchers looked at what happens when the two particles have unequal charges (though still of the same sign).
They found that, when two particles with unequal charges in a 1:1 electrolyte solution approach each other, the nanoparticle with the stronger charge will polarize the metal core of the nanoparticle with the weaker charge, meaning a majority of the electrons in the core will bunch up on one side of the core. This causes that nanoparticle to have a slight positive charge on one side and a slight negative charge on the other side. The polarization-induced nonuniform charges on the nanoparticle's core can cause the two unequally charged nanoparticles to attract one another, even though the nanoparticles have the same overall charge sign. The observation that the attraction occurs only between spherical metal unequally charged nanoparticles, and not between metal slabs, indicates the importance of curvature and the presence of a central core for this counterintuitive result.
Besides being an interesting theoretical discovery, the results could also be very useful when applied to gold nanoparticles, which are being developed for a variety of medical applications such as cancer treatment and drug delivery. Gold nanoparticles have a strong affinity for some biological surfaces, such as phospholipid membranes, which enclose cells. In the new study, the researchers demonstrated that negatively charged gold nanoparticles are generally repelled from the negatively charged surfaces of phospholipid membranes. However, under certain conditions the force between the gold nanoparticles and the membranes becomes attractive. The researchers plan to further explore these effects and their implications in future research.
"The mechanism that we described might also be important for understanding the stability of suspensions of biological particles," Levin said. "The usual method of stabilizing suspensions of nanoparticles is through like-charge repulsion—basically synthesizing particles with surface charge so that they repel one another and do not stick together. Here we show, however, that if the suspension is sufficiently polydisperse in size and charge, like-charged nanoparticles can actually attract one another, sticking together and precipitating."
One of the challenges the researchers confronted during their work was quantitatively modeling the new results, as conventional methods are highly computationally expensive. To address this problem, the researchers developed a modified numerical approximation method for calculating forces between nanoparticles that works orders of magnitude faster than conventional methods. The new method also offers advantages for studying the forces between metal nanoparticles and biological membranes, as well as for exploring more complicated solutions. The researchers plan to further investigate both areas in the future.
"In our group, we have an extensive line of research on colloidal systems, which ranges from simulations to theory," Levin said. "So far we have examined theoretically the effects of polarization on metal particles in 1:1 electrolyte. Since the correlation effects in such solutions are not very strong, such systems are susceptible to our theoretical treatment. However, in more complicated solutions such as 3:1 electrolyte, the correlation effects between ions will be very important and our theoretical tools will not suffice. In this case we are developing simulation methods to study the interaction between metal nanoparticles."
More information: Alexandre P. dos Santos and Yan Levin. "Like-Charge Attraction between Metal Nanoparticles in a 1∶1 Electrolyte Solution." Physical Review Letters. DOI: 10.1103/PhysRevLett.122.248005
Journal information: Physical Review Letters
© 2019 Science X Network