Quantum physicists succeed in controlling energy losses and shifts

Quantum computers need to preserve quantum information for a long time to be able to crack important problems faster than a normal computer. Energy losses take the state of the qubit from one to zero, destroying stored quantum information at the same time. Consequently, scientists all over the globe have traditionally worked to remove all sources of energy loss—or dissipation—from these machines.
Dr. Mikko Mottonen from Aalto University and his research team have taken a different approach. "Years ago, we realized that quantum computers actually need dissipation to operate efficiently. The trick is to have it only when you need it," he explains.
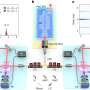
In their paper to be published on 11 March 2019 in Nature Physics, scientists from Aalto University and the University of Oulu demonstrate that they can increase the dissipation rate by a factor of thousand in a high-quality superconducting resonator on demand—such resonators are used in prototype quantum computers.
"The quantum-circuit refrigerator that we recently invented was the key to achieve this tunability of dissipation. Future quantum computers need a similar feature to be able to control energy loss on demand," says Mottonen.
According to the first author of the work, Dr. Matti Silveri, the results of most scientific significance were unexpected.
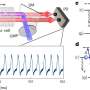
"To our great surprise, we observed a shift in the resonator frequency when we turned on the dissipation. Seventy years ago, Nobel winner Willis Lamb made his first observations of small energy shifts in hydrogen atoms. We see the same physics, but for the first time in engineered quantum systems," explains Silveri.
Lamb's observations were revolutionary at that time. They showed that modeling the hydrogen atom alone was not enough; electromagnetic fields must be accounted for, even though their energy is zero. This phenomenon is now confirmed also in quantum circuits.
The key to the new observation was that dissipation, and hence the energy shift, can be turned on and off. Control of such energy shifts is critical for the implementation of quantum logic and quantum computers.
"Building a large-scale quantum computer is one of the greatest challenges of our society," Mottonen says.
More information: Broadband Lamb shift in an engineered quantum system, Nature Physics (2019). DOI: 10.1038/s41567-019-0449-0 , www.nature.com/articles/s41567-019-0449-0
Journal information: Nature Physics
Provided by Aalto University