New research area: How protein structures change due to normal forces
Proteins made in our cells are folded into specific shapes so they can fulfill their functions. Scientists have discovered the static structures of over 100,000 proteins, but how they change in response to forces on the cell, like muscle contractions, is largely unknown. Matthias Wilmanns and colleagues at the European Molecular Biology Laboratory in Hamburg, Germany, developed methods to study the structure of a protein "strain absorber" as it changes during muscle contractions. They will present their work at the 63rd Biophysical Society Annual Meeting, to be held March 2—6, 2019 in Baltimore, Maryland.
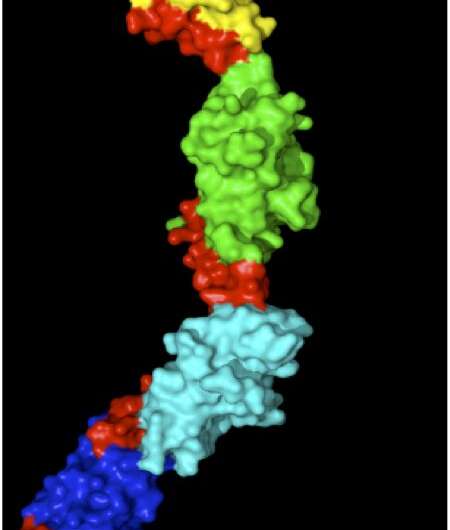
Each muscle unit has a series of highly organized protein rods that are pulled to overlap when a muscle contracts or are pulled apart when a muscle is stretched. Myomesin is a protein that stabilizes and organizes these rods, acting to absorb the strain on stretched muscles to prevent the muscle units from breaking apart. Wilmanns, in collaboration with Matthias Rief's group at the Technical University of Munich, used atomic force microscopy to stretch and measure individual myomesin molecules. Myomesin became 2.5 times longer under force, and their high resolution structure showed this was due to slinky-like linkers in the protein that allow it to stretch without unfolding. However, a key question remains on demonstrating that these mechanisms apply under physiological conditions as well. To address this question, Wilmanns and colleagues are now designing experiments to visualize the changes in myomesin inside muscle cells using super high-resolution imaging.
"Muscle is a good model for looking at how its proteins respond to force, because it experiences extraordinarily high forces, but we have small forces all over the body," explained Wilmanns. "Now we have methods sensitive enough to measure very small forces, so we can start looking at the behavior of different proteins that respond to very small forces. At present there is so little known about mechanisms of molecular elasticity in proteins."
Provided by Biophysical Society