Researchers obtain an important chemical compound

Since the discovery of the first homoleptic metal carbonyl complex Ni(CO)4 more than 130 years ago, scientists try to obtain further such compounds formed from a carbon monoxide molecule and a metal that are important for basic research as well as applications. The last new compound of this type to be bottled, the Co(CO)5 cation, was reported in 2003. However, extensive research in the gas phase has shown that far more metal carbonyl complexes than those known to date should exist, including the chromium hexacarbonyl cation.
A team led by the chemists Prof. Dr. Ingo Krossing from the University of Freiburg and Prof. Dr. Frank Breher from the Karlsruhe Institute of Technology were able to prepare this compound in common solvents and fill it into a bottle as a stable compound in crystalline form. They present their results in the journal Nature Communications.
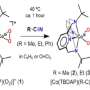
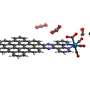
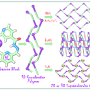
"For over fifty years, researchers have been trying to prepare this highly studied and dynamic floppy molecule as a substance that can be bottled," explains Krossing. The research groups from Freiburg and Karlsruhe produced a combination of a strong oxidizing agent [NO]+ and a weakly coordinating anion, which enables the oxidation of Cr(CO)6, chromium hexacarbonyl, and isolated its room temperature stable radical salt. Normally [NO]+ leads to a coordination of the released nitrogen monoxide, but the researchers were able to suppress this with suitable reaction conditions. In the end, they succeeded in filling the stable compound, which, as found in the experiments, has several isomeric structures close in energy, into bottles as a solution and as crystals.
"Since we have used standard laboratory equipment and Schlenk techniques as well as common solvents for our method," said the professor. "It can now be used in all chemical laboratories and can therefore be used for a wider range of applications.
More information: Jan Bohnenberger et al. Stable salts of the hexacarbonyl chromium(I) cation and its pentacarbonyl-nitrosyl chromium(I) analogue, Nature Communications (2019). DOI: 10.1038/s41467-019-08517-2
Journal information: Nature Communications
Provided by Albert Ludwigs University of Freiburg