Upending a fundamental reaction in organic chemistry—discovery of a new nucleophilic substitution reaction
Nucleophilic substitution is a class of chemical reactions encountered throughout organic chemistry, including those used to manufacture common petrochemical and pharmaceutical products. Its underlying mechanism was discovered 82 years ago by the British chemists Edward Hughes and Christopher Ingold, who showed that an electron-rich chemical species, called a nucleophile, "attacks" and replaces an electron-poor fragment of an organic molecule, called a leaving group.
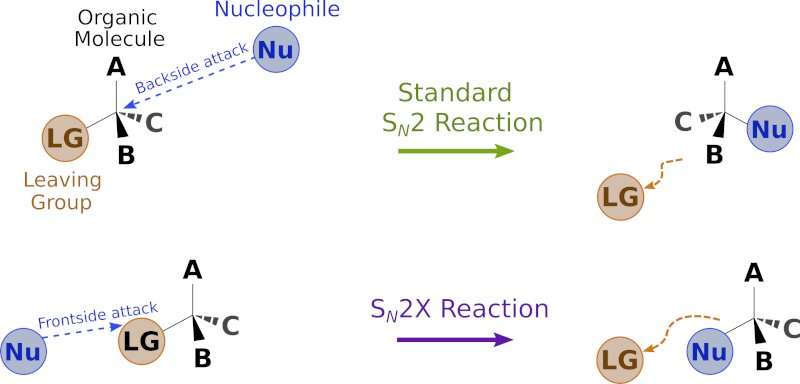
One of the main types of nucleophilic substitution reactions, called SN2, involves the nucleophile attacking and the leaving group departing at the same time. Hughes and Ingold first made the observation, subsequently confirmed by generations of chemists, that SN2 reactions all seemed to occur via "backside attack," whereby the nucleophile joins the organic molecule at a location opposite to the leaving group.
Although SN2 reactions were believed to be understood, a new variant has been found by a group of scientists in Singapore. In an upcoming paper due to be published in the journal Science, a research group led by Professor Choon-Hong Tan of Nanyang Technological University (NTU) reports that SN2 reactions can also occur via "frontside attack," whereby the nucleophile approaches the molecule on the same side as the leaving group.
One of the key characteristics of a standard SN2 reaction is that the nucleophile, in attempting a backside attack, is blocked by other parts of the molecule. This phenomenon, called "steric hindrance," imposes strict limits on how rapidly SN2 reactions can happen. By contrast, the newly discovered reaction, which the researchers call SN2X, occurs via frontside attack and is not prone to steric hindrance.
The discovery of the SN2X reaction involved a considerable amount of chemical detective work. "We had to design our experiment to exclude the possibilities of several other types of reactions, as well as carefully checking that the reaction byproducts were consistent with our interpretation," commented Xin Zhang, an NTU graduate student who was the first author on the paper.
The absence of steric hindrance in SN2X means that certain reactions in organic chemistry can be performed more efficiently than previously believed. "In the paper, we demonstrated the SN2X reaction in a specially chosen set of reactions – enantioselective reactions of sterically hindered tertiary halides," explained Professor Tan. "But now that one example has been found, it seems very likely that others will follow. The revision of such a foundational part of organic chemistry means that many reactions, which chemists thought we understood, might now have to be re-examined. This could have wide-ranging implications throughout the field."
More information: "An enantioconvergent halogenophilic nucleophilic substitution (SN2X) reaction" Science (2019). science.sciencemag.org/cgi/doi … 1126/science.aau7797
Journal information: Science