Harnessing light for a solar-powered chemical industry

New technology that harnesses sunlight to drive chemical reactions is paving the way for a more sustainable chemical manufacturing industry, one of the globe's biggest energy users.
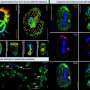
RMIT University researchers have developed a nano-enhanced material that can capture an incredible 99% of light and convert it to power chemical reactions.
As well as reducing the environmental impact of chemical manufacturing, the innovation could one day be used to deliver technologies like better infrared cameras and solar-powered water desalination.
Published today in ACS Applied Energy Materials, the research addresses the challenge of finding alternative energy sources for chemical manufacturing, which accounts for about 10% of global energy consumption and 7% of industrial greenhouse gas emissions.
In the US, chemical manufacturing uses more energy than any other industry, accounting for 28% of industrial energy consumption in 2017.
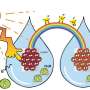
While photo catalysis—the use of light to drive chemical reactions—is growing in the industry, efficiency and cost remain significant obstacles to wider take-up.
Lead investigator Associate Professor Daniel Gomez said the new technology maximised light absorption to efficiently convert light energy into chemical energy.
"Chemical manufacturing is a power hungry industry because traditional catalytic processes require intensive heating and pressure to drive reactions," Gomez, an ARC Future Fellow in RMIT's School of Science, said.
"But one of the big challenges in moving to a more sustainable future is that many of the materials that are best for sparking chemical reactions are not responsive enough to light."
"The photo catalyst we've developed can catch 99% of light across the spectrum, and 100% of specific colours.
"It's scaleable and efficient technology that opens new opportunities for the use of solar power—moving from electricity generation to directly converting solar energy into valuable chemicals."
Nano-tech for solar power
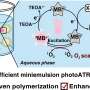
The research focused on palladium, an element that's excellent at producing chemical reactions but usually not very light responsive.
By manipulating the optical properties of palladium nanoparticles, the researchers were able to make the material more sensitive to light.
While palladium is rare and expensive, the technique requires just a miniscule amount—4 nanometres of nano-enhanced palladium is enough to absorb 99% of light and achieve a chemical reaction. An average human hair, for comparison, is 100,000 nanometres thick.
Beyond chemical manufacturing, the innovation could be further developed for a range of other potential applications including better night vision technology by producing more light-sensitive and clearer images.
Another potential use is for desalination. The nano-enhanced material could be put in salty water then exposed to sunlight, producing enough energy to boil and evaporate the water, separating it from the salt.
Gomez, who leads the Polaritonics Lab at RMIT, said the new technology could significantly increase the yield in the emerging photo-catalysis sector, with leading firms currently producing about 30kg of product each day using light as the driving force.
"We all rely on products of the chemical manufacturing industry—from plastics and medicines, to fertilisers and the materials that produce the colours on digital screens," he said.
"But much like the rest of our economy, it's an industry currently fuelled by carbon.
"Our ultimate goal is to use this technology to harness sunlight efficiently and convert solar energy into chemicals, with the aim of transforming this vital industry into one that's renewable and sustainable."
More information: Timothy U. Connell et al, Directing energy into a sub-wavelength non-resonant metasurface across the visible spectrum., ACS Applied Energy Materials (2019). DOI: 10.1021/acsaem.8b01704
Provided by RMIT University