Wildfire ash could trap mercury
In the summers of 2017 and 2018, heat waves and drought conditions spawned hundreds of wildfires in the western U.S. And in November, two more devastating wildfires broke out in California, scorching thousands of acres of forest, destroying homes and even claiming lives. Now, researchers studying ash from recent California wildfires report in ACS' Environmental Science & Technology that burned material in forests might help sequester mercury that otherwise would be released into the environment.
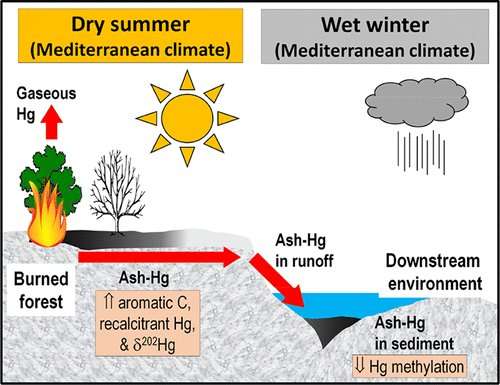
Forest fires release substantial amounts of mercury into the atmosphere, but some mercury remains behind in wildfire ash. The fine, powdery nature of this material makes it prone to runoff into aquatic environments, where microbes can convert the element into highly toxic methylmercury. Yet the levels and fate of mercury in wildfire ash, which can be black or white in color, are unclear. Black ash is generated from low-intensity burning, whereas white ash results from higher burn intensities. Martin Tsz-Ki Tsui, Xiangping Nie and colleagues wanted to examine mercury levels and reactivity in black and white ash from two recent California wildfires.
When the researchers analyzed mercury levels, taking into account the amount of organic matter lost to combustion, they found that black ash, white ash and unburned vegetation from the wildfire areas contained similar amounts of mercury. However, most ash samples contained higher amounts of mercury that was in a chemically unreactive form than did the unburned samples. Stream water mixed with white or black ash for 4 or 12 weeks had lower amounts of mercury and methylmercury compared with water containing unburned vegetation. The researchers propose that black carbon, or charcoal, in the ash could bind mercury and keep it in a non-reactive form that is not released into water.
More information: Origin, Reactivity, and Bioavailability of Mercury in Wildfire Ash, Environmental Science & Technology (2018). pubs.acs.org/doi/abs/10.1021/acs.est.8b03729
Journal information: Environmental Science & Technology
Provided by American Chemical Society