New technique to identify phloem cells aids in the fight against citrus greening
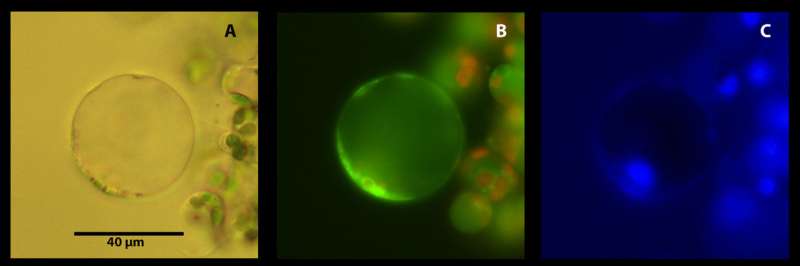
Crops worldwide are increasingly vulnerable to pandemics, as diseases hitch rides on global flows of people and goods, hopping from continent to continent. Phloem diseases such as citrus greening are one particularly devastating group of plant diseases that have been wreaking economic havoc globally. However, these diseases can be difficult to study, as phloem cells are relatively inaccessible and difficult to isolate. In work presented in a recent issue of Applications in Plant Sciences, Dr. Ed Etxeberria and colleagues at the University of Florida Citrus Research and Education Center present a new technique for identifying phloem cells in plant tissue.
"Because of their inaccessible nature, phloem-limited diseases are virtually impossible to cure or treat in planta, and therefore, pose immense risks both in economic and biological terms. In economic terms, mild phloem diseases can cause significant reductions in agricultural output by reducing quality and quantity of the host agricultural commodity. In the worst cases, they can spell the end of established industries, as is the case of the citrus industry in Puerto Rico," said Dr. Etxeberria, corresponding author of the study. "In biological terms, these diseases threaten the very survival of the affected species."
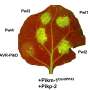
In order to fight the devastation of phloem diseases, researchers must understand in detail the way these pathogens deform the plant phloem cells known as sieve elements and companion cells. Today, many basic questions remain unanswered about how diseases like citrus greening impact these phloem cells, which are essential for plant nutrient transport. "We are interested in finding out whether the signal produced by the [citrus greening] bacteria, that directs the physiological changes in the sieve element and is mediated by the companion cells, is genetic or chemical." As a first step in answering this question, phloem cells would have to be isolated. However, this presents a technical challenge, as phloem cells constitute less than 1% of total cells, are buried deep within plant tissues, and are interspersed with other cell types.
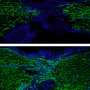
Existing techniques for the identification of phloem cells rely on the presence of phloem-specific proteins called forisomes. However, forisomes are only found in the phloem cells of plants in the bean family, limiting the application of this method. The technique presented here takes advantage of the distinctive anatomy of phloem cells by using organelle-specific dyes and fluorescent microscopy. For example, phloem cells called sieve element lack a nucleus and vacuole, but possess parietal mitochondria, so these cells become apparent when tissue is stained with the organelle-specific Hoechst 3342, Neutral Red, and MitoTracker Green and visualized with a fluorescent microscope. This method is applicable well beyond citrus, because it relies on the anatomy of phloem cells, rather than protein markers that vary from species to species. That means it could be used to understand not only citrus greening but a wide variety of phloem diseases such as cucurbit yellow vine disease, corn stunt disease, and onion yellow dwarf disease.
While studying phloem diseases is by far the most pressing application for this technique, and was the motivation for this study, identification of phloem cells could also help with the study of other botanical questions. "Identifying phloem cells can help in other areas of phloem physiology such as characterizing the location of membrane-bound carriers or channels, their location throughout the plant, and their properties depending on their distribution in source or sink tissues," said Dr. Etxeberria. Additionally, because it involves digestion of the cell wall, "this method can be used to study membrane properties such as ion fluxes, membrane electrical properties, and biophysics of membrane elasticity—a study not possible with intact cells."
Using established biological methods like cell wall digestion, organelle-specific staining, and fluorescent microscopy, Dr. Etxeberria and colleagues have developed a technique to accurately isolate phloem cells across plants. While this technique has wide applications, it will immediately be put into service in understanding and fighting the devastating phloem diseases causing crop failures worldwide.
More information: Prabhjot Kaur et al, Identification of sieve elements and companion cell protoplasts by a combination of brightfield and fluorescence microscopy, Applications in Plant Sciences (2018). DOI: 10.1002/aps3.1179
Journal information: Applications in Plant Sciences
Provided by Botanical Society of America