Scientists discover stability in hybrid photoelectric nanomaterials
A team of Siberian scientists and foreign colleagues calculated the parameters that influence the intensity of the reaction between carbon nanotubes and phthalocyanines—complex nitrogen-containing compounds. Hybrid constructions based on them are considered as new materials for solar cell batteries, sensors and optic devices. The work was published in Applied Surface Science.
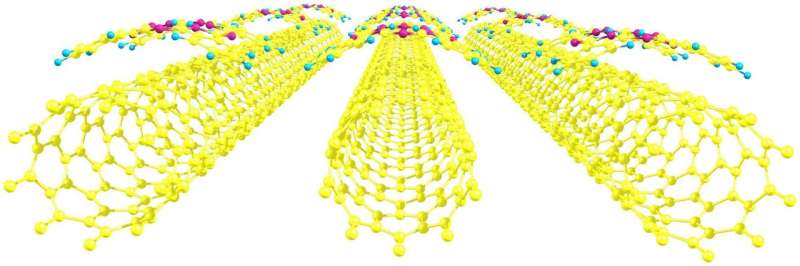
Many new materials for photoelectric devices combine two non-organic and organic chemical elements. The first may be represented by carbon nanotubes—hollow cylinders with walls made of hexagons with atoms of carbon at vertexes. The organic part may be comprised of heterocyclic compounds such as phthalocyanines. These substances consist of several carbon rings bound with nitrogen atoms and are able to form complexes with metals. This combination is not arbitrary: Cyclic molecules donate electrons, and carbon nanostructures accept them. Continuous transitions secure electrical conductivity in a photoelectric material.
"One of the issues with hybrids like that is low stability of the chemical bond between the organic and non-organic parts. As a result, phthalocyanines become quite mobile on the surface of carbon nanotubes. This is a disadvantage, as in this case, certain properties are not attributed to the material homogeneously," said Pavel Krasnov, a senior research associate at the Institute for Nanotechnologies, Spectroscopy, and Quantum Chemistry, Siberian Federal University.
In the course of the work, the scientists considered the dependence of nanotubes-phthalocyanines bond stability on a number of parameters, such as diameter and form of the carbon nanostructure, nature of the metal forming a complex with the organic component, and so on. As the result of the quantum-mechanical modeling, the researchers found which parameters should be changed and how to increase bond stability to its maximum.
The chemists discovered that the position of a phthalocyanine molecule relative to a tube was an important factor. The strongest bond was observed when a cross-shaped organic molecule "hugged" the cylinder, like a sloth hugging a thick branch. The type of a metal that forms a complex with phthalocyanine also plays an important role: In the cobalt-zinc-copper range the bond strength decreases. Another interesting relation was discovered between the orientation of the grid of hexagons and its size. For nanotubes with diameter less than 10.5 Å (one angstrom is 10-10m), the most stable bond is formed in the case of an "armchair" configuration when the connections of hexagons in the grid that are perpendicular to the axis of the tube are chair-shaped. In case of bigger diameter, the most advantageous shape is "zigzag."
"The discovered relations will help to create target hybrid nanostructures with the highest binding capacity between carbon nanotubes and phthalocyanines. These materials may be used in many areas, but their main purpose is photoelectronics," concludes Pavel Krasnov.
More information: Pavel O. Krasnov et al. Interaction of metal phthalocyanines with carbon zigzag and armchair nanotubes with different diameters, Applied Surface Science (2018). DOI: 10.1016/j.apsusc.2018.06.282
Provided by Siberian Federal University