Core electron topologies in chemical bonding
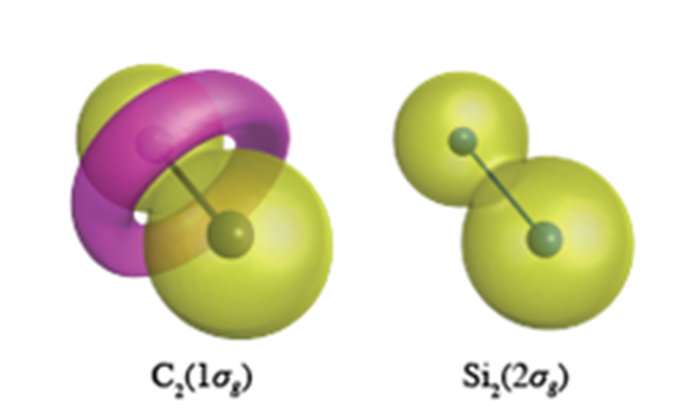
YNU researchers have solved the age-old mystery of why silicon cannot replace carbon in organic compounds. A new benchmark quantum chemical calculation of C2, Si2, and their hydrides reveals a qualitative difference in the topologies of core electron orbitals of organic molecules and their silicon analogues. The researchers propose other elements with carbon's propensity to reshape their core electron nodal structures upon chemical bonding.
Since the discovery of silicon and Wöhler's success in the mid-19th century with synthesizing organic compounds, Wöhler himself was among the first to suggest replacing carbon by silicon in organic compounds. It became clear in the early 20th century that silicon does not have a chemistry similar to carbon, and dreams of silicon-based life only survive in science fiction. We know empirically that carbon has the capability to form a variety of unsaturated compounds, which silicon does not. However, the root cause of why only carbon has this capability has remained a mystery.
Quantum chemical calculations of unprecedented accuracy carried out at YNU reveal that core electrons (which were not supposed to participate in chemical bonding) have a very different role in the unsaturated compounds of carbon and silicon. Carbon has the propensity to alter the topology (nodal structure) of its core electrons, which, for C2, results in the formation of a torus-like ring in the 1σg orbital formed of C1s electrons (see Figure). Si2, however, maintains the spherical like core orbitals centered at each atomic site in all its molecules. This flexibility of carbon's core orbitals allows carbon to form a cornucopia of different valence bond structures, whereas silicon is restricted to bond structures orthogonal to the atomic like spherical core orbitals.
The impact of this discovery is far-reaching. Core electrons have thus far been assumed more or less inert, but perhaps it becomes necessary to reassess their contribution to chemical bonding—at least in the case of unsaturated bonds. Finally, the study suggests that other elements, such as nitrogen, phosphorous, and fluorine, exhibit similar flexibility to modify their core electron topologies, and thus, exhibit similarly rich chemistries.
The paper, "Core Electron Topologies in Chemical Compounds: Case Study of Carbon versus Silicon," is published in Angewandte Chemie International Edition vol 57(24) on June 6th, 2018.
More information: Daisuke Yoshida et al. Core Electron Topologies in Chemical Compounds: Case Study of Carbon versus Silicon, Angewandte Chemie International Edition (2018). DOI: 10.1002/anie.201713108
Journal information: Angewandte Chemie International Edition
Provided by Yokohama National University