Chemical engineers advance olefins production through computational modeling
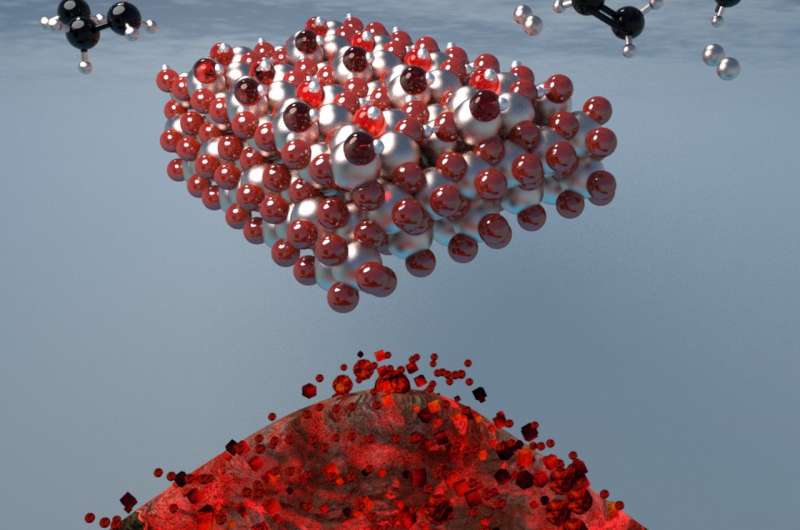
Olefins are simple compounds of hydrogen and carbon but represent the building blocks of chemistry, and are vital for the synthesis of materials from polymers and plastics to petrochemicals. However, olefin production requires the use of nonrenewable fossil fuels, energy intensive "cracking" facilities, and limited production control.
New research from the University of Pittsburgh's Swanson School of Engineering has introduced a method to effectively screen different catalysts that convert light alkanes to olefins. With light alkanes being abundant in the Marcellus and Utica shale reserves, this methodology may provide a more economical solution for olefins production.
Their research, "Structure-Activity Relationships in Alkane Dehydrogenation on γ-Al2O3: Site-Dependent Reactions" was recently featured on the cover of ACS Catalysis. Lead investigator is Giannis Mpourmpakis, the Bicentennial Alumni Faculty Fellow and Assistant Professor of Chemical and Petroleum Engineering at the Swanson School, and co-authors Mudit Dixit, Ph.D. and Pavlo Kostetskyy, postdoctoral fellow at Northwestern University who earned his Ph.D. in Dr. Mpourmpakis' CANELa lab.
"The tremendous success and vast reserves of shale gas have transformed the chemical market and made methane and light alkanes a versatile feedstock for value-added chemicals production," Dr. Mpourmpakis explained. "One of the most promising routes toward olefins is the dehydrogenation of alkanes on metal oxides, which is the chemical removal of molecular hydrogen from a hydrocarbon. But this process is energy intensive since it involves high temperatures and the dehydrogenation reaction mechanism is not well understood. As a result, any progress on olefins production relies on lengthy and expensive trial-and-error experiments in the lab."
According to Dr. Mpourmpakis, determining exactly how the alkane dehydrogenation activity depends on the exact type of different sites present on the surface of metal oxides has been difficult, in part because of the diversity of the many sites. His lab applied computational chemistry and mathematical modeling tools to predict how alkane dehydrogenation mechanisms and catalytic activity change on the different sites of the oxides.
"Being able to computationally screen these metal oxide surfaces and identify the exact catalytic active sites greatly limits trial-and-error experimentation in the lab," Dr. Mpourmpakis said. "We now have a better tool to develop active catalysts for alkane-olefin conversion, which could be a game-changer in the petrochemical and polymer industries."
More information: Mudit Dixit et al, Structure–Activity Relationships in Alkane Dehydrogenation on γ-Al2O3: Site-Dependent Reactions, ACS Catalysis (2018). DOI: 10.1021/acscatal.8b03484
Journal information: ACS Catalysis
Provided by University of Pittsburgh