Nanodiamonds as photocatalysts
Climate change is in full swing and will continue unabated as long as CO2 emissions continue. One possible solution is to return CO2 to the energy cycle: CO2 could be processed with water into methanol, a fuel that can be easily transported and stored. However, the reaction, which is reminiscent of a partial process of photosynthesis, requires energy and catalysts. Developing light-active photocatalysts from abundant, easily obtained materials would enable green, climate-neutral solar fuels.
A candidate for such photocatalysts are so-called diamond nanomaterials—these include nanostructured carbon foams with a high surface area, and tiny nanocrystals of a few thousand carbon atoms that are soluble in water and look like a black slurry. In order for these materials to become catalytically active, however, they require UV light excitation. Only this spectral range of sunlight is rich enough in energy to transport electrons from the material into a free state. Only then can solvated electrons be emitted in water and react with the dissolved CO2 to form methanol.
However, the UV component in the solar spectrum is not very high. Photocatalysts that could also use the visible spectrum of sunlight would be ideal. This is where the work of HZB-scientist Tristan Petit and his cooperation partners in DIACAT comes in: Modelling the energy levels in such materials shows that intermediate stages can be built into the band gap by doping with foreign atoms. Boron, a trivalent element, appears to be particularly important.
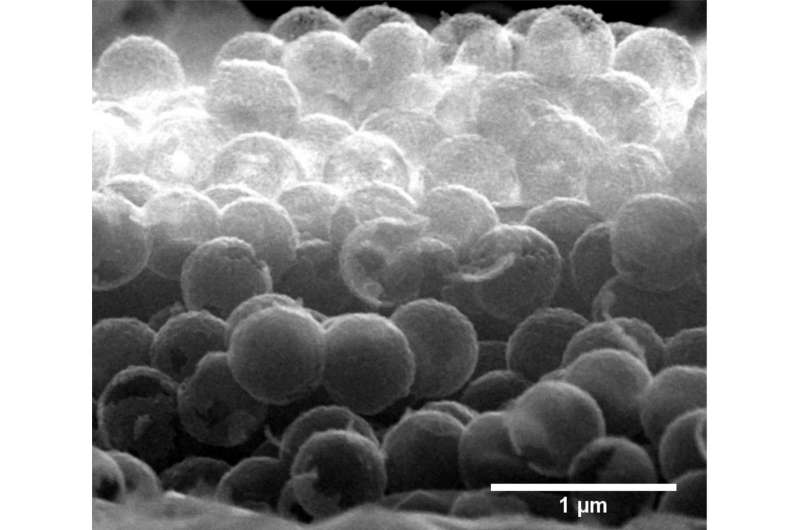
Petit and his team therefore investigated samples of polycrystalline diamonds, diamond foams and nanodiamonds. These samples had previously been synthesized in the groups of Anke Krüger in Würzburg and Christoph Nebel in Freiburg. At BESSY II. The researchers used X-ray absorption spectroscopy to measure the unoccupied energy states where electrons could possibly be excited by visible light. "The boron atoms present near the surface of these nanodiamonds actually lead to the desired intermediate stages in the band gap," explains Ph.D student Sneha Choudhury, first author of the study. These intermediate stages are typically very close to the valence bands, and thus do not allow the effective use of visible light. However, the measurements show that this also depends on the structure of the nanomaterials.
"We can introduce and possibly control such additional steps in the diamond bandgap by specifically modifying the morphology and doping," says Tristan Petit. Doping with phosphorus or nitrogen could also offer new opportunities.
More information: Sneha Choudhury et al, Combining nanostructuration with boron doping to alter sub band gap acceptor states in diamond materials, Journal of Materials Chemistry A (2018). DOI: 10.1039/c8ta05594g
Journal information: Journal of Materials Chemistry A
Provided by Helmholtz-Zentrum Berlin für Materialien und Energie