Insulator becomes conductor at the push of a button
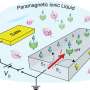
Ionic liquids are important in scientific research because they can apply a lot of charge over a surface. Physicists from Leiden University have now found that the charging process of ionic liquids depends purely on opposite charges attracting each other. Chemical reactions are sometimes involved, but not essential.
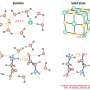
In studies with electricity, physicists often want to apply as much charge as possible over a surface for research on material properties or to generate a huge electric pulse in one go. Ionic liquids are remarkably suited for this because they apply charge through ions. These charged particles keep a more stable charge than their solid-state equivalent, electrons. Within an ionic liquid, opposite ions accumulate on both sides of a surface, which gets charged as a result. The charging process is so effective that it can make an insulating surface conductive.
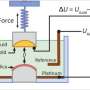
Physicist Jan van Ruitenbeek, together with Hasan Atesci and others from his group, study the charging process within an ionic liquid by cooling it down to around -100 °C and making sure there is no water or oxygen present. In these conditions, there is no electrochemistry. Yet the process continued, albeit more slowly than at room temperature. The team concluded that only electrostatic processes—attraction between opposite charges—are necessary for the charging process. Although chemical reactions are involved at room temperature, they are apparently not essential.
A promising application for ionic liquids is a 'super' version of a capacitor—a so-called supercapacitor. Capacitors can be useful because they release a stored charge in one powerful electric pulse. "A capacitor stores electricity on two plates, one positively and one negatively charged," says Van Ruitenbeek. "To store a lot of charge, you have three options: enlarge the surfaces, reduce the distance between them or increase the voltage. With ionic liquids, the distance can be as small as the size of the ions, so about a nanometer. Plus, ionic liquids are very stable, so you can apply a large voltage without inducing chemical processes, which would limit the lifetime of the capacitor."
More information: Hasan Atesci et al. On the Formation of a Conducting Surface Channel by Ionic-Liquid Gating of an Insulator, Annalen der Physik (2018). DOI: 10.1002/andp.201700449
Provided by Leiden Institute of Physics