Chemists accelerated vinyl sulphides reaction 10 times
A team of scientists created and tested a more efficient catalyst for obtaining vinyl sulphides. These compounds may be used for the development of new materials and stabilization of gold and silver nanoparticles. The article about the study was published in the Catalysis Science & Technology journal.
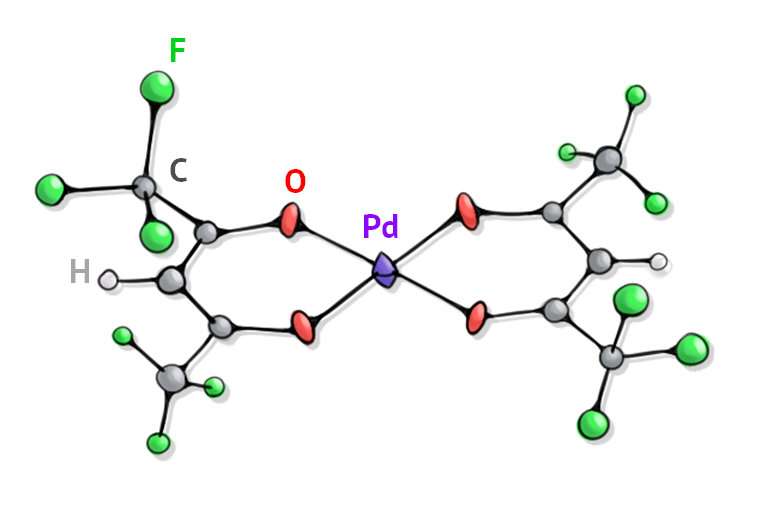
The authors of the work created and tested an new catalyst that consists of the central atom of palladium and radicals containing fluorine atoms that are connected to it. Using this substance, the chemists accelerated the reaction for obtaining vinyl sulphides (sulfur-based compounds) 10 times. The catalyst prevented the formation of insoluble by-products which increased the speed of the reaction.
"Our group changed the mechanism of the reaction and managed to increase its efficiency 10 times while keeping the reaction mix homogenous. As a rule, a reaction between palladium and one of initial substances causes the deposition of insoluble palladium thiolate. The use of the new catalyst will help preserve the homogeneity of the solution preventing the formation of additional deposits and therefore accelerating the vinyl sulphides reaction," explains professor Dr. Victor Khrustalev, a co-author of the work, and head of the inorganic chemistry department at RUDN.
The chemists established the main structural features of several developed compounds and discovered a connection between a substance's structure and catalytic activity. The scientists demonstrated that the use of the catalyst in lower concentrations (up to 10 times lower than described above) may speed up several vinyl sulphide reactions up to 100 times. However, the volumes of obtained substances in this case were considerably smaller, and numerous by-products prevented their extraction.
"Although all features of the new methods haven't been studied in full yet, we undoubtedly showed its applicability for a wide range of initial substances for the first time. Our work can mark a start for the development of systems with zero catalyst efficiency loss to be used widely in real life and opening new possibilities for modern chemistry," says Victor Khrustalev.
Vinyl sulphides can damage the structure of DNA in tumor cells and therefore destroy them. They are also used for creating substances with antimicrobial properties. Vinyl sulphides are able to form long strings (polymers) that are resistant to mechanical load, high temperatures, and the influence of highly active chemical substances. Therefore, materials based on vinyl sulphides are used to filtrate acids and alkalis and in the manufacture of pipes and condensers. They can also prevent the settling of precious metal nanoparticles (gold and silver) in colloid solutions, such as used in the production of medicinal drugs.
More information: Dmitry B. Eremin et al. Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand, Catalysis Science & Technology (2018). DOI: 10.1039/C8CY00173A
Provided by RUDN University