Tiny brushes that make surfaces slippery may not work as intended, study finds
A type of molecular surface thought to be extremely slippery may not stay that way under all conditions, according to new UChicago and Argonne research in Science.
The study by scientists from the Institute for Molecular Engineering at the University of Chicago and Argonne National Laboratory may have implications for those trying to tap these surfaces for new technologies, such as joint replacements or anti-fogging surfaces.
Scientists have become very interested in a type of molecular formation called a polyelectrolyte brush over the past decade, said study coauthor and IME Director Matt Tirrell, because they are thought to make surfaces slippery. These molecules, which look like a field of tiny hairs standing on end when charged, are kept straight because the negative charges along each brush repel each other. Similar molecules line our joints and our gastrointestinal tracts.
But to date, studies all looked at these brushes while immersed in pure water or water with ions with only +1 charges. Many conditions in the real world, such as inside the human body, involve exposure to liquids with multivalent ions—those with +2 or +3 charges, like calcium and magnesium, instead of just +1.
When the team decided to investigate how the brushes performed in such salty liquids, they saw the slipperiness drop off steeply.
"All it takes is minute amounts of these ions to completely change the structure," said study coauthor Juan de Pablo, the Liew Family Professor in Molecular Engineering. "We might expect to see some change, but to see such dramatic changes with such small amounts was a surprise."
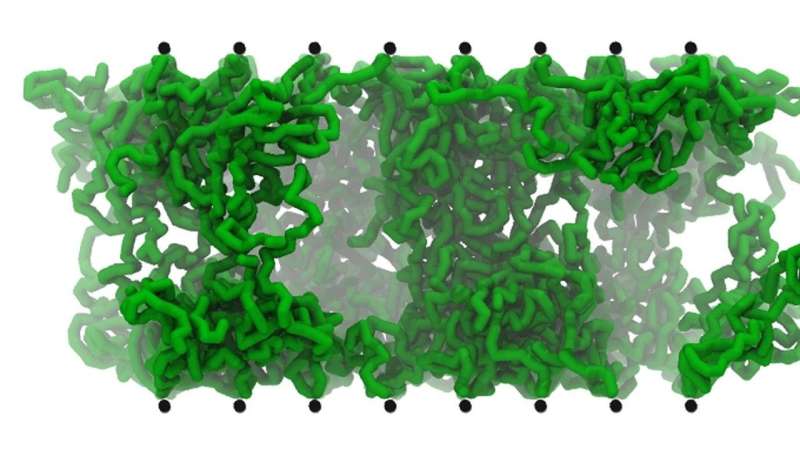
When Nick Jackson, a Maria Goeppert Mayer Fellow at Argonne, simulated the reactions, they could see the drama play out at the molecular level.
"These multivalent salts just collapse the whole thing," Tirrell said. "The normal forces between the surfaces instead get attracted to one another, and the brushes get sticky and shrink down into little blobs."
The effect also worsens when the brushes are squeezed together—another common condition in the real world.
It's a striking effect, the scientists said, and it's a concern for scientists and engineers trying to make the brushes into technology. "It's possible that these polyelectrolyte brushes are not really fundamentally responsible for joint lubrication," Tirrell said, or that there are other effects at play that we don't yet fully understand.
The simulation was partially run on Blues, a high-performance computing cluster operated by the Laboratory Computing Resource Center at Argonne National Laboratory.
More information: J. Yu et al. Multivalent counterions diminish the lubricity of polyelectrolyte brushes, Science (2018). DOI: 10.1126/science.aar5877
Journal information: Science
Provided by University of Chicago