Systematically studying slippery surfaces
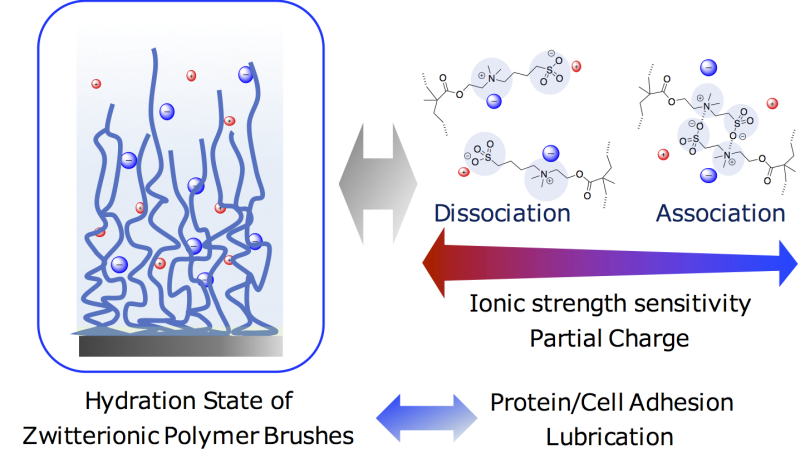
Betaines are materials with both a positively charged functional group and a negatively charged functional group linked by an alkyl chain spacer. This combination of functional groups causes betaines to strongly attract water, which makes them useful for water retention. Betaines prevent cell dehydration in organisms and also play roles in some reactions. Commercially, betaines are used in DNA amplification and chemical synthesis. Betaine groups can be included in polymer brushes, which are polymers that are tethered to a surface. The resulting polymer brushes are attractive for use in lubrication and anti-fouling applications because the hydrated betaine groups prevent adhesion of other materials. However, the hydration states of polymer brushes are still poorly understood.
It is known that the length of the alkyl chain spacer between the charged functional groups in a betaine affects its hydration state. A Japanese collaboration led by Kyushu University recently investigated the effects of the charged group spacer length of betaines in polymer brushes on their hydration state to provide a clearer picture of the relationship between the structure and properties of these materials.
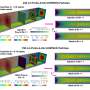
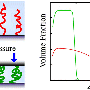
The researchers first synthesized a series of polymer brushes containing alkyl chain spacers of different lengths on silicon wafers. The polymer brushes were then characterized by a range of techniques. In particular, neutron reflectivity was used to probe the hydration states of the polymer brushes. The results of polymer brush characterization revealed that only some parameters depended on the length of the alkyl chain spacer. Water uptake by the polymer brushes exposed to water or humid air did not depend on chain spacer length.
"We found that the polymer brushes swelled in humid air and water because the hydration of the charged betaine groups led to the decrease of the interfacial free energy between water and the brushes," says Yuji Higaki, an assistant professor at Kyushu University's Institute for Materials Chemistry and Engineering (IMCE) / International Institute for Carbon-Neutral Energy Research (WPI-I2CNER).
"Our findings reveal how the charged group spacer length of betaines modulates the interaction of polymer brushes with water," states team leader Atsushi Takahara, a professor at IMCE and WPI-I2CNER.
The team's results should aid the development of lubricating and anti-fouling surfaces for use in marine environments or under physiological conditions.
The article "Effect of Charged Group Spacer Length on Hydration State in Zwitterionic Poly(sulfobetaine) Brushes" was recently published in Langmuir .
More information: Yuji Higaki et al, Effect of Charged Group Spacer Length on Hydration State in Zwitterionic Poly(sulfobetaine) Brushes, Langmuir (2017). DOI: 10.1021/acs.langmuir.7b01935
Journal information: Langmuir
Provided by Kyushu University, I2CNER