Putting gas under pressure

Understanding gas flames' response to acoustic perturbations at high pressure should make next-generation turbines safer and more efficient.
Soldiers marching lockstep across a bridge can cause the structure to collapse if the rhythm of their step matches the bridge's natural vibration frequency. Combustion engineers must consider a similar effect when designing the gas turbines used in electricity generation and aero-engines.
Just as soldiers' feet can cause bridge sway to reach the point of destruction, a gas turbine can be damaged, or even explode, if heat and pressure fluctuations produced by the flame couple with the acoustics of the combustion chamber. At a lesser degree, this'thermoacoustic instability hampers efficient combustion, increasing noise and pollution emissions.
Predicting and preventing thermoacoustic instabilities remains challenging for the design of a gas turbine. To improve the models used, Deanna Lacoste from KAUST's Clean Combustion Research Center and her colleagues have measured the stability of gas flames at elevated pressure.
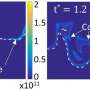
Investigation of the flame's response to acoustic forcing, uses a parameter called flame transfer function (FTF), says Francesco Di Sabatino, a Ph.D. student in Lacoste's team. The FTF is derived from experimental measurements of the flame's response to sound waves. But these experiments are usually run at atmospheric pressure, whereas real gas turbines reach pressures of up to 30 bar.
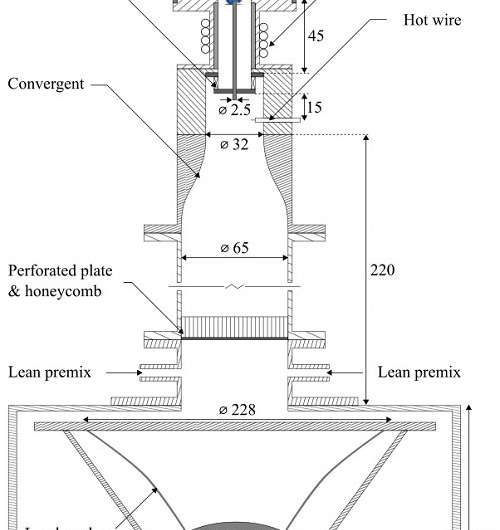
Lacoste, Di Sabatino and their colleagues systematically investigated the effect of pressure on methane and propane gas flames. "Our experiments show the FTF at atmospheric pressure is different to the FTF at elevated pressure," says Di Sabatino. For both methane and propane gas flames, pressure had a particularly strong effect when the loudspeaker produced acoustic perturbations of 176 Hz.
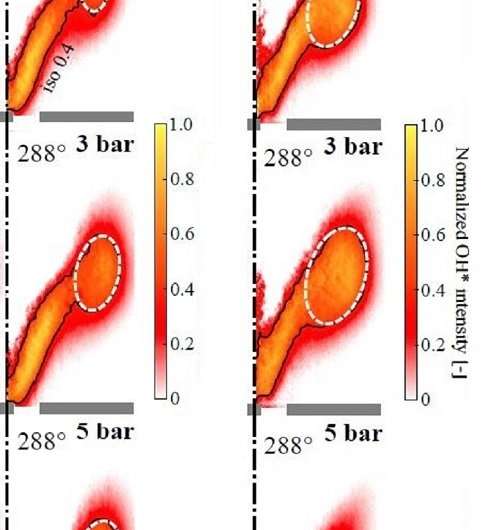
More information: Francesco Di Sabatino et al. Effect of pressure on the transfer functions of premixed methane and propane swirl flames, Combustion and Flame (2018). DOI: 10.1016/j.combustflame.2018.03.011