Exploring the chemistry of nuclear explosions
To understand fallout formation from a nuclear explosion, it's important to look at the gas phase of metal oxides within the device.
Lawrence Livermore National Laboratory (LLNL) scientists have developed a plasma-flow reactor to experimentally simulate the late cooling of post-detonation fireballs where temperature drops below 10,000 K. They investigate the formation of nanoparticles from gas phase atoms to unravel the chemical fractionation processes uranium and other chemical elements go through during fireball condensation. The research appears in a recent edition of the journal Scientific Reports.
The researchers hope to better understand the interconnection between chemical reactions and micro-physical processes (e.g. nucleation, condensation, growth, etc.) on time scales that are relevant to fallout formation.
"We showed a quantifiable link between recovered particle size distribution and gas phase chemical kinetics—a consideration that is absent in the current fallout formation models." said Batikan Koroglu, LLNL postdoctoral researcher and lead author of the paper.
The formation of nanoparticles from gas phase is an important topic for many areas of chemistry and physics. The formation of particulates following a nuclear explosion is a special case involving the rapid condensation of material from an initial high-temperature plasma state. Past studies investigated nuclear debris samples to understand the fate and transport of post-detonation material in the atmosphere. However, the interplay between equilibrium thermodynamics, chemical kinetics and oxygen fugacity (local environment) is still unknown for uranium subjected to extreme temperature conditions.
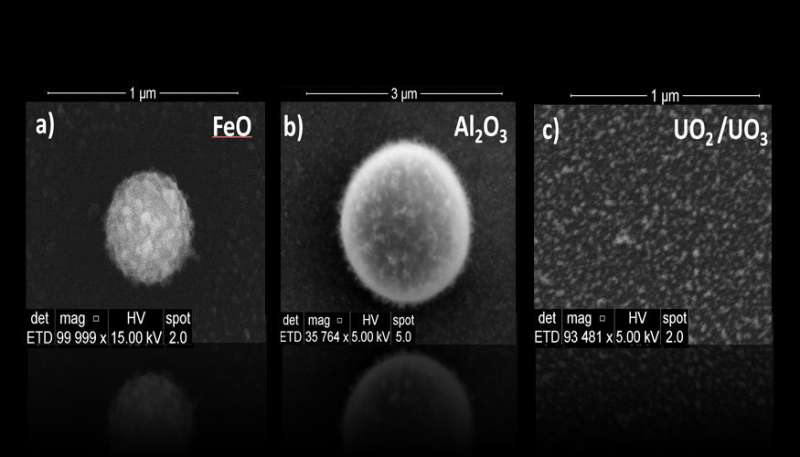
The team's plasma-flow reactor allowed them to monitor the gas phase chemical evolution of three types of metals (iron, aluminum and uranium) leading to nanoparticle formation using in-situ optical spectroscopy and ex-situ electron microscopy measurements. These three metals were chosen because their oxides exhibit very distinct volatilities.
"Understanding the gas phase chemistry of recombination reactions is necessary to accurately describe the condensation patterns observed during the fast cooling of a nuclear explosion," Koroglu said.
More information: Batikan Koroglu et al. Gas Phase Chemical Evolution of Uranium, Aluminum, and Iron Oxides, Scientific Reports (2018). DOI: 10.1038/s41598-018-28674-6
Journal information: Scientific Reports
Provided by Lawrence Livermore National Laboratory