Porous silica protects nickel catalyst
By wrapping nickel nanoparticles in a protective shield of porous silica, A*STAR researchers have developed a highly active and robust catalyst that could help to produce methane from biomass.
Biomass is a potentially carbon neutral feedstock to make fuels or other useful chemicals. Through a process called gasification, biomass is converted to a mixture, known as syngas, comprising carbon monoxide, carbon dioxide and hydrogen. Syngas can be turned into a range of other chemicals, including methane, which may be used as a transportation fuel or town gas, or burned to generate electricity.
Various catalysts convert syngas to methane. Nickel is one of the most common, due to its high activity and moderate cost, and it is typically supported on another material such as alumina or silica. But the catalyst can become deactivated during this high temperature methanation reaction, either through a build-up of carbon called coking, or by a process called sintering in which catalyst particles clump together. Moreover, any traces of sulfur compounds in the syngas can very quickly switch off nickel's catalytic activity, so syngas must go through an expensive cleaning process to remove sulfur before methanation.
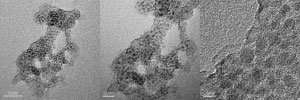
Luwei Chen of the A*STAR Institute of Chemical and Engineering Sciences and colleagues have now embedded nickel nanoparticles in porous silica, which allows gases to access the catalyst, but prevents the problems that cause deactivation.
They prepared the catalyst by mixing particles of nickel hydroxide with tetraethyl orthosilicate. After further processing, they activated the nickel by reacting it with hydrogen at 600 degrees Celsius, forming particles that contained about 40 per cent nickel by weight. The researchers tested their catalyst with syngas derived from a gasification process, and with a simulated syngas, both of which contained sulfur. Using techniques such as transmission electron microscopy, X-ray diffraction and thermal gravimetric analysis, they found that the catalyst experienced very little sintering or coking during the reaction, unlike a commercial catalyst that was tested using the same syngas samples. "Porous silica protects by isolating each particle, to prevent sintering," says Chen.
The nickel-silica catalyst also withstood sulfur impurities for three times as long as its commercial rival before deactivating. Improving the sulfur resistance of the catalyst in this way could lead to significant cost savings in the syngas cleaning process. The researchers are now collaborating with IHI, a Japanese engineering company, to scale up their synthesis of the catalyst, and the methanation process.
More information: Hiroyuki Kamata et al. Dispersed and high loading Ni catalyst stabilized in porous SiO 2 matrix for substituted natural gas production, Catalysis Today (2017). DOI: 10.1016/j.cattod.2017.03.003