Straightforward technique allows for accurate computer simulations of calcium signaling
Calcium is essential for our bodies to function. Calcium ions enable cells to communicate with one another, allowing neurons to interact, muscles to contract, and the heart's muscle cells to synchronize and beat. To better understand these processes, in which calcium ions interact with biological molecules such as proteins, researchers often use computer simulations. But accurate models are challenging and computationally expensive.
"If you have the wrong model of calcium, it will simply not work," said Pavel Jungwirth of the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences in Prague. "Most of the models that are available are not accurate enough to capture the important features of the calcium ion."
In this week's issue of The Journal of Chemical Physics, however, Jungwirth's research group demonstrates how a straightforward modification in a computer model leads to highly accurate simulations, which serve as powerful tools for studying a range of biological processes. "I believe that we have the best of the simple models of calcium in the world at the moment," Jungwirth said.
Calcium ions travel from cell to cell as messengers. When they reach a cell, they bind to a molecule, such as a protein, triggering a cascade of chemical responses. But due to the ion's watery environment, simulating exactly how calcium binds is difficult.
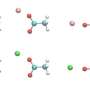
The calcium ion, which is doubly positively charged, interacts strongly with the oxygens of the surrounding water molecules. These oxygens have a partial negative charge (as in the molecule of water) and the oxygen atom attracts the electrons of the bonds more effectively. The electrostatic forces between calcium and water induce the water molecules to rearrange themselves around the ion. The calcium ion also forces the electrons in the water molecule to shift, a phenomenon called electronic polarization.
Most simulations incorporate the rearrangement of water molecules. But because calculating exactly how electrons move requires too much computing power, they don't take electronic polarization into account. Without electronic polarization, Jungwirth said, simulations involving calcium are inaccurate.
Typically, interactions with water molecules work to pull a calcium ion away from the molecule it's trying to bind with, like in a molecular tug of war. If a simulation doesn't fully take these effects into account, it overestimates how strongly the calcium binds, producing ions that can't unbind, which is unrealistic.
A few years ago, however, Alexei Stuchebrukhov and Igor Leontyev proposed a solution: Lower the electric charge of the ions in the simulations. It turns out that scaling the charge by a factor of about 0.75 mimics the effect of electronic polarization. Such a simple scaling also doesn't add any extra computational burden.
"It's almost a miracle," Jungwirth said. "We know it's not a perfect solution, but maybe it solves 90 percent of the problem."
Previously, Jungwirth's team tested the strategy by modeling the relatively simple interaction between calcium and chloride ions. To check if the simulations were accurate—and if the scaling worked—they blasted real calcium chloride solutions with neutrons. By measuring how those neutrons scattered off the aqueous calcium chloride, the researchers deduced its structure and compared the data with the simulations.
In the new study, the researchers tested their model with carboxylic groups—molecular groups found in proteins, and thus more relevant for biology. After also adjusting the charge of the carboxylic group, they again showed that their simulations matched up very well with data from neutron scattering experiments.
Because carboxylic groups are simple compared to, say, an entire protein, the researchers could also describe the calcium interactions using accurate but computationally expensive electronic structure calculations. By comparing these calculations to the simulations, they again confirmed the accuracy of their models.
These tests show that the new model can simulate calcium interactions with almost any protein, Jungwirth said. The researchers have also developed an analogous model that works for calcium interactions with phospholipids at the cell membrane. The next step, he said, is to do the same with DNA and RNA molecules. And further along, the researchers plan to develop a similar model for magnesium, another important signaling ion with its own unique challenges.
More information: "Calcium ions in aqueous solutions: Accurate force field description aided by ab initio molecular dynamics and neutron scattering," Journal of Chemical Physics (2018). DOI: 10.1063/1.5006779
Journal information: Journal of Chemical Physics
Provided by American Institute of Physics