Graphene oxide nanosheets could help bring lithium-metal batteries to market
Lithium-metal batteries—which can hold up to 10 times more charge than the lithium-ion batteries that currently power our phones, laptops and cars—haven't been commercialized because of a fatal flaw: as these batteries charge and discharge, lithium is deposited unevenly on the electrodes. This buildup cuts the lives of these batteries too short to make them viable, and more importantly, can cause the batteries to short-circuit and catch fire.
Now, researchers at the University of Illinois at Chicago have developed a solution to this problem in the form of a graphene-oxide coated 'nanosheet' that, when placed in between the two electrodes of a lithium-metal battery, prevents uneven plating of lithium and allows the battery to safely function for hundreds of charge/discharge cycles. They report their findings in the journal Advanced Functional Materials.
"Our findings demonstrate that two-dimensional materials—in this case, graphene oxide—can help regulate lithium deposition in such a way that extends the life of lithium-metal batteries," said Reza Shahbazian-Yassar, associate professor of mechanical and industrial engineering in the UIC College of Engineering and corresponding author of the paper.
Lithium-metal batteries are so useful because of their high-energy density and relatively light weights compared with conventional batteries. However, over the course of many charge-discharge cycles, lithium builds up unevenly on the battery's lithium metal electrode in a branching or 'dendritic' pattern and ultimately causes the battery to go dead. If the dendrites grow through the electrolyte solution and make contact with the other electrode, then the battery may experience a catastrophic event—in other words, an explosion or fire.
In lithium-ion batteries, a separator is placed in the electrolyte. Usually made of a porous polymer or glass ceramic fibers, the separator allows lithium ions to flow through while keeping the other components blocked to prevent electrical shorts, which can lead to fires.
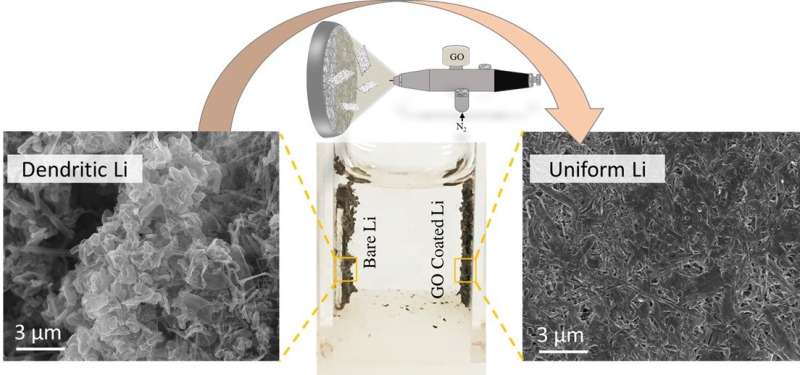
Reza and colleagues used a modified separator in a lithium-metal battery to modulate the flow of lithium ions to control the rate of lithium deposition and see if they could prevent dendrites from forming. They spray-coated a fiberglass separator with graphene oxide, producing what they called a nanosheet.
Using scanning electron microscopy and other imaging techniques, the researchers showed that when the nanosheet was used in a lithium-metal battery, a uniform film of lithium formed on the lithium electrode's surface, which actually improves battery function and makes the battery much safer, said Tara Foroozan, a graduate student in the UIC College of Engineering and first author on this study.
Molecular simulations, led by a team of researchers from Texas A & M University, suggested that the lithium ions become temporarily bonded to the graphene oxide, and then diffuse through areas of nanoscopic defects in the sheet. This delays the passage of lithium ions enough to prevent the formation of dendritic deposition of lithium on the electrode.
"The nanosheet slows the passage of lithium ions enough to allow for more uniform plating on lithium ions across the surface of the electrode, which helps preserve battery life," said Reza.
Results of phase-field modeling computations led by Farzad Mashayek, professor and head of mechanical and industrial engineering in the UIC College of Engineering and an author on the paper, indicated that graphene oxide can also mechanically suppress the growth of lithium dendrites.
"We show that two-dimensional graphene oxide materials are able to impede the formation of dendrites by changing the rate of lithium-ion diffusion as they pass through the graphene oxide layers," said Shahbazian-Yassar. "This method has very high potential for industrial application and scalability."
More information: Tara Foroozan et al, Synergistic Effect of Graphene Oxide for Impeding the Dendritic Plating of Li, Advanced Functional Materials (2018). DOI: 10.1002/adfm.201705917
Journal information: Advanced Functional Materials
Provided by University of Illinois at Chicago