Bridging tumor moats with potent drug delivery particles
Despite herculean efforts, cancer remains a formidable disease, with each malignant subtype responding differently to therapeutics. One hurdle specific to treating solid tumors is a protective layer called an extracellular matrix that can prevent chemotherapeutic agents from penetrating the tumor's core. Scientists now report results in ACS' Chemistry of Materials showing that, by cloaking anti-cancer drugs in a specially designed particle, they could target and destroy tumor cells deep inside a malignant mass in vitro.
For tumors that can't be extracted with surgery, radiation and chemotherapy are the treatments of choice, but both can involve serious side effects due to a lack of specificity: They'll kill healthy cells along with malignant ones. Researchers have long known that, thanks to the unique blood vessel architecture surrounding tumors, nanoparticles can easily pass into the cancer zone, offering a potential route for the specific delivery of chemotherapies to cancer cells. However, efforts to exploit this phenomenon have fallen short, with experimental drug-loaded particles failing because they can't get through the dense extracellular matrix or they lose the therapeutic payload en route to the tumor's interior. Alejandro Baeza, C. Jeffrey Brinker, Maria Vallet-Regi and colleagues addressed this shortcoming by developing a brand-new type of particle.
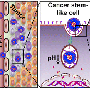
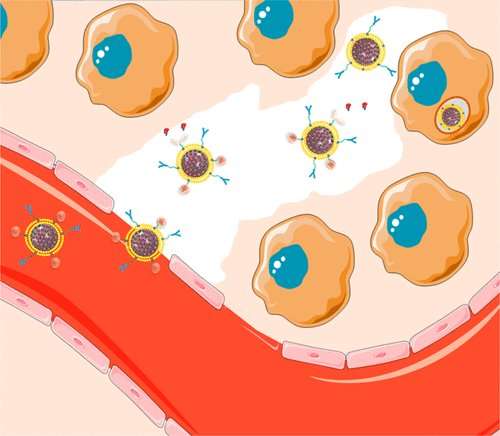
The researchers created a "protocell," a nanoparticle that can carve through the extracellular matrix, delivering cell-killing doses of drug to the deepest tumor regions. To develop the protocell, the team started with a mesoporous silica skeleton with a high internal surface area that can contain a large amount of drug. They surrounded this skeleton with a lipid bilayer outfitted with an array of tools to help the protocell deliver its drug arsenal to the desired locale, including enzymes that cleave collagen, a major component of the tumor's extracellular matrix. The protocell also features pH-sensitive ligands that trigger the release of the drug upon entry into the relatively acidic interior of a cell, ensuring the medication is only delivered where needed. The researchers tested the protocells in a 3-D cell culture model of a solid tumor, showing that the protocell penetrates and destroys malignant cells better than drug-loaded protocells without the enhanced toolkit.e potential to one day treat cancer and other diseases in the female reproductive tract.
More information: María Rocío Villegas et al. Multifunctional Protocells for Enhanced Penetration in 3D Extracellular Tumoral Matrices, Chemistry of Materials (2017). DOI: 10.1021/acs.chemmater.7b03128
Abstract
The high density of the extracellular matrix in solid tumors is an important obstacle to nanocarriers for reaching deep tumor regions and has severely limited the efficacy of administrated nanotherapeutics. The use of proteolytic enzymes prior to nanoparticle administration or directly attached to the nanocarrier surface has been proposed to enhance their penetration, but the low in vivo stability of these macromolecules compromises their efficacy and strongly limits their application. Herein, we have designed a multifunctional nanocarrier able to transport cytotoxic drugs to deep areas of solid tumors and once there, to be engulfed by tumoral cells causing their destruction. This system is based on mesoporous silica nanocarriers encapsulated within supported lipid bilayers (SLBs). The SLB avoids premature release of the housed drug while providing high colloidal stability and an easy to functionalize surface. The tumor penetration property is provided by attachment of engineered polymeric nanocapsules that transport and controllably unveil and release the proteolytic enzymes that in turn digest the extracellular matrix, facilitating the nanocarrier diffusion through the matrix. Additionally, targeting properties were endowed by conjugating an antibody specific to the investigated tumoral cells to enhance binding, internalization, and drug delivery. This multifunctional design improves the therapeutic efficacy of the transported drug as a consequence of its more homogeneous distribution throughout the tumoral tissue.
Journal information: Chemistry of Materials
Provided by American Chemical Society