Scientists analyze the chemical bonds that shape proteins
An international group of scientists including visiting foreign professor from RUDN University Kamran Makhmudov has analyzed chemical bonds in proteins based on sulfur and other elements from the 16th group of the periodic table. Such atoms are called chalcogens, and the bonds are known as chalcogen bonds. The results were published in Dalton Transactions, and will be presented at the International Chugaev Conference on Coordination Chemistry to be held from October 2 to 6 in Nizhny Novgorod (Russia).
"Over the past two years, more than 100 research papers on chalcogen bonding were published every year in the scientific database Web of Science," Kamran Makhmudov, the lead author of the work explains. "Interest in this topic has been growing exponentially for a decade, but surprisingly, there was no generalized article on the use of chalcogen bonds in synthesis, catalysis and materials design relevant for modern chemistry. We believe that this perspective that systematizes existing information on the applications of chalcogen bonding will fill this gap and draw more attention to this new growing field of research."
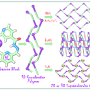
The arrangement of atoms inside a molecule is determined by covalent bonds. They are formed when atoms share pairs of electrons. When it comes to protein molecules, covalent interactions between atoms determine the primary structure of the molecule (the "chain" of amino acids).
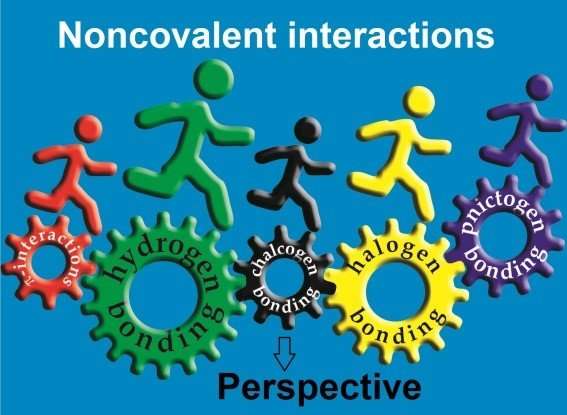
Along with covalent bonds between atoms and polyatomic particles, there are noncovalent interactions. Noncovalent bonds (aerogenic, halogenic, chalcogenic, pnictogenic, tetrel and icosagen) are formed by the elements of the 13th to 18th groups in the periodic table: hydrogen, halogens (such as chlorine, bromine, fluorine and iodine), chalcogens (elements of the oxygen and sulfur subgroup), pnictogens (arsenic, antimony, bismuth). The atoms of these chemical elements have a positive electrostatic potential. In other words, these atoms get a positive charge that attracts negatively charged atoms of chemical elements. This is the working principle of Lewis acids—their acid center attracts negatively charged molecules (enriched by electrons which give them this negative charge).
"It is due to noncovalent interactions that clusters of atoms or molecules can exist in a condensed state—in the form of a liquid or a solid. These interactions play a large role when we deal with polymers," said Kamran Makhmudov. "In particular, different protein complexes are combined through noncovalent interactions either with each other or with nucleic acids to form ribosomes, chromatin, viruses, or with lipids to make up lipoprotein membranes. Thus, noncovalent interactions form the basis for important biological structures and their role in biology is particularly important."
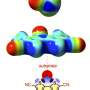
Scientists have discovered how chemical elements from the chalcogen group form noncovalent chemical bonds. This group includes oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po), and artificially produced radioactive Livermorium (Lv).
Chalcogen bonding is one of the types of noncovalent interactions. A chalcogen atom is tied to a molecule by covalent bonds, but it has one or more positively charged areas. Due to the attraction of positive to negative charges, the chalcogen atom attaches to other parts of the molecule that have negatively charged areas. This is how the chalcogen bond is formed. This is one of the mechanisms of protein molecule folding which holds its shape.
Chalcogen bonding is usually observed in substances in the solid state. But in several studies, the chalcogens were also active in a solution. This is a very important property, since it makes chalcogens useful for analytical chemistry and medicine. Moreover, it is already known that the chalcogen bonding (mainly the interaction between sulfur and oxygen) plays an important role in biological systems. Scientists believe that we should start thinking about including chalcogens in drug design. With the help of multiple chalcogen bonds between the centers of sulfur, selenium and tellurium, we can create nanotubes that will contain other molecules.
"We hope that these examples and related discussion will draw more attention to this exciting field of practical application of chalcogen. Moreover, we can expect chalcogen bonding to be recognized by the International Union of Pure and Applied Chemistry (IUPAC) in the near future," the scientist concluded.
More information: Kamran T. Mahmudov et al, Chalcogen bonding in synthesis, catalysis and design of materials, Dalton Trans. (2017). DOI: 10.1039/c7dt01685a
Journal information: Dalton Transactions
Provided by Peoples' Friendship University of Russia