Multifunctional nano-sized drug carriers based on reactive polypept(o)ides
Nano-sized carrier systems have medical application in improving pharmacologic properties of bioactive agents. For many therapeutic approaches, it is important that the carrier system can stably incorporate the cargo during circulation without inducing aggregation, while cargo should ideally only be released after successful cellular uptake. These requirements have thus far only been met by chemistry approaches with nanoparticles that are difficult to characterize. Consequently, clinical translation of these systems has been very difficult to achieve.
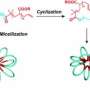
Chemists from Mainz and collaborators have demonstrated that reactive polypept(o)ides constitute ideal building blocks to control morphology and function of carrier systems in a simple but precise manner. Polypept(o)ides (polysarcosine-block-polypeptide copolymers) have emerged as interesting hybrid materials for drug carrier systems since they combine protein resistance and high water solubilty of polysarcosine with the stimuli responsiveness, intrinsic multifunctionality, and secondary structure formation of polypeptides.
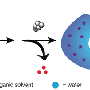
In this cooperative work, the researchers showed for the first time that the formation of β-sheets by the synthetic polypeptide segment can be exploited to deliberately manipulate the morphology of polymeric micelles (Klinker K et al. Angew. Chem. Int. Ed. 2017, 56 (32), 9608-9613 & Angew. Chem. 2017, 129 (32), 9737–9742), which enables the synthesis of either spherical or worm-like micelles from the same block copolymer. By employing reactive groups in the polypeptide segment of the block copolymer, micelles can be core cross-linked by dithiols, resulting in bio-reversible disulfide bonds. Due to a difference in redox potential, disulfides are considered stable extracellularly, while they are rapidly reduced to free dithiols intracellularly, which leads to a disintegration of the carrier system and release of the cargo.
"In this way, a variety of nanocarriers with different functions becomes readily accessible from one single block copolymer and a very selective post-polymerization step. This modular approach to nanoparticles with different function and morphology addresses important questions with good comparability, such as the influence of size and shape on in vivo circulation times, biodistribution, tumor accumulation, cell uptake and therapeutic response since the same starting material is used," says Matthias Barz.
First in vivo experiments have already demonstrated that these core stabilized micellar nanocarriers exhibit stable circulation behavior, thus indicating that interactions with serum components or blood vessels are absent. Only by ensuring that no unspecific interactions occur within the complex biological setting, cellular uptake in desired specific cell populations seems feasible. The therapeutic potential of the described nanoparticle platform will be further investigated with regards to immunotherapy of malignant melanoma within the SFB 1066.
More information: Kristina Klinker et al. Secondary-Structure-Driven Self-Assembly of Reactive Polypept(o)ides: Controlling Size, Shape, and Function of Core Cross-Linked Nanostructures, Angewandte Chemie International Edition (2017). DOI: 10.1002/anie.201702624
Journal information: Angewandte Chemie International Edition
Provided by Universitaet Mainz