August 4, 2017 report
Ultrasonic vibrations force a polymer to be a semiconductor
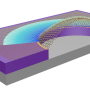
![Design of mechanochemically generated conjugated polymer. (A) Insulating CBE mechanophores connected with π systems, designed to rearrange to continuously extended conjugation under force. (B) Thermally unstable CBEs with sp2 carbon substituents at the 3 and 4 positions. (C) Ladderene-based mechanophore, which would undergo tandem mechano-cycloreversions to give conjugated oligoene. (D) Natural [5]-ladderane fatty acid (5) from anammox bacteria. (E) Design of mechanically active polyladderene via ROMP of ladderene. Credit: (c) Science (2017). DOI: 10.1126/science.aan2797 Ultrasonic vibrations force a polymer to be a semiconductor](https://scx1.b-cdn.net/csz/news/800a/2017/598463de6e3f9.jpg)
(Phys.org)—A team of researchers at Stanford University has used mechanical force to transform a molecule from one form to another—from a nonconductive state into a semiconductor. In their paper published in the journal Science, the group describes the process they developed and possible applications.
As the researchers note, using mechanical force to change a molecule from one form to another (by prying open their bonds) has been a popular research subject over the past decade, leading to a new field now known as mechanochemistry. In this new effort, the researchers used a physical force to "unzip" a nonconducting polymer, transforming it into a semiconductor.
In their work, the team studied chunks of cyclobutanes to learn more about their structure. In their natural state, they exist as a polyladderene molecule with the appearance of stairs leading from a low point to a high point, and walls holding them in place. The team thought that if they could pull the walls apart, effectively unzipping the staircase, they could transform it into a zig-zag-looking polymer known as a polyacetylene, which is a semiconductor.
The cyclobutanes were placed in a solution and subjected to sonic waves exerting opposing forces on the molecule, causing it to unzip and stretch out into nearly a flat structure (into alternating C=C double bonds). The group reports that the solution, which was initially clear, slowly changed to blue, and eventually became dark as it was filled with a mesh of nanowires. The researchers note that the material could be used as a means for measuring stresses in other materials. It could also be used to mimic human senses in a robot because it is able to use a mechanical force to convert a material into a wire capable of carrying an electronic signal. But before that can happen, the team acknowledges that more work needs to be done to make the structures simpler, as they are now they are too complex for industrial applications.
More information: Zhixing Chen et al. Mechanochemical unzipping of insulating polyladderene to semiconducting polyacetylene, Science (2017). DOI: 10.1126/science.aan2797
Abstract
Biological systems sense and respond to mechanical stimuli in a complex manner. In an effort to develop synthetic materials that transduce mechanical force into multifold changes in their intrinsic properties, we report on a mechanochemically responsive nonconjugated polymer that converts to a conjugated polymer via an extensive rearrangement of the macromolecular structure in response to force. Our design is based on the facile mechanochemical unzipping of polyladderene, a polymer inspired by a lipid natural product structure and prepared via direct metathesis polymerization. The resultant polyacetylene block copolymers exhibit long conjugation length and uniform trans-configuration and self-assemble into semiconducting nanowires. Calculations support a tandem unzipping mechanism of the ladderene units.
Journal information: Science
© 2017 Phys.org