August 15, 2017 report
Pure optical detection of spikes for the ultimate brain machine interface
(Phys.org)—Brain-machine interfaces (BMIs) are basically gimmicks. The reason you don't hear so much about them these days is because, in the fullness of time, significant tangible benefit to a user has flat out failed to materialize. Simply stated, neither prickly microelectrode arrays, harrowing optogenetic reworks to our physiology, nor tattooing our brains with toxic fluorescents WILL ever give us what we need. On the other hand, if you can watch native spikes bubble unmolested through axon tracts from afar, sans any of the aforementioned hazards, you might be onto something.
While any serious brain researcher must be fully aware of these truths at some gut a level, any collective admission as such would require several basic underpinnings of the field to be jettisoned. For starters, this means letting go of the idea that spikes are fully described by the strictly electrical epiphenomena researchers amplify on their oscilloscopes. In other words, representing axons as equivalent circuits irreversibly dissipating their spike energy through various impedances falls short. Fortunately, a critical mass of researchers have now developed tools to probe the larger intrinsic physics of the spike. The goal is to develop a more general theory of excitability in cells which can explain all the observed physical changes (such as dimension, pressure and temperature). Their secret sauce, that which will eventually yield brain devices we covet, is label-free optical detection of mechanical spikes.
Although there is a long history of work in this field, several recent papers suggest we are finally beginning to understand this physics. The first paper uses the tried and true method of fiber optical interferometry to detect the nanometer scale changes in optical path length that occur when cells spike. The second paper manages to extract 0.2 nm scale excursions in the cell envelope during spikes using image subtraction and denoising techniques. Finally, a third set reports on the huge micron-scale displacements in spiking Chara plant cells, and revisits the intriguing question of what happens when spikes traveling in opposite directions collide.
Can we make practical BMIs with interferometers?
For widespread practical BMIs to ever become a reality they are probably going to need to be small. Classic Michelson interferometers, the kind every physics major recreates at some point in a laboratory course, have not generally been associated with compactness or configurability. While suitable for things like disproving the aether or glimpsing gravitational waves using massive optical legs, Michelson interferometers aren't always the first choice for biologic experiments. Instead, the Mach-Zehnder interferometer is often used because each of its well-separated light paths is only traversed once, making it much more versatile. Mach-Zehnder modulators can now be built as monolithic integrated circuits that have high-bandwidth electro-optic amplitude and phase responses over a multiple GHz frequency range.
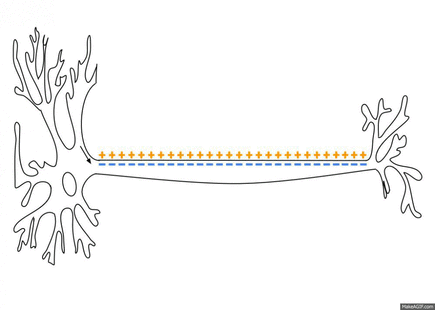
Despite the seeming advantages of the Mach Zehnder, author Digant Dave from the first paper said they use the Michelson interferometer for their experiments because the common path topology gives very high axial sensitivity. In particular, they can measure displacements of less than 0.1nm in an in vitro cell preparation. The probe beam spot size is ~4.5 μm and high SNR is achieved by sandwiching neurons between two pieces of glass. The recorded optical pulses ranged from 20 to 300 ms (mostly under 50ms), which is a bit longer than the 5 to 7 ms range for the spikes they recorded via patch clamping.
I asked Dave how an an in vivo 2-D nerve scanning implementation of his in vitro setup might theoretically be made. He said that the fiber tips themselves could be as small as 1mm and be used in either of two modes: either raster scan the probe beam, or acquire 2-D images while scanning the input light source wavelength. At a millimeter in diameter each, I would think it should be possible to thread several such probes into the ventricular system of the brain in order to scan the vast axon tracts lining the walls of the 3rd and 4th ventricles. Just beneath the cerebellum are several natural vents which circulate CSF to equilibrate pressure. In particular, the Foramens of Magendi and Lushka would be ideally suited for the purpose.
Pending further miniaturization, much of the hardware for signal processing and perhaps even optical beam prep may still have to be remain closely apposed or tethered outside the body. Of more immediate concern than hardware however, would be the effects of myelin on the signal. To date, most of the studies have been done using bare axons or plant cells that have been denuded of their cell wall. Myelin might absorb or otherwise attenuate mechanical and thermal pulses, or quite possibly it could have an amplifying effect on other variables like pressure. For example, when the Chara cells were 'plasmalysed', as reported in the third paper, to eliminate the cell wall and the associated turgor pressure afforded by it, the smaller 100nm scale displacements were converted to micron scale displacements.
I asked Digant what he thought about the prospect of measuring displacements without interferometers as was reported in the second paper mentioned. While he noted that 0.2nm sensitivity was very impressive for a standard bright field scope, he observed that these measurements were made laterally in the cell envelope and required significant averaging of hundreds of frames. The authors were also able to simultaneously patch clamp the cells to compare amplitude and phase of the electrically recorded spike, however, this itself may complicate the mechanical measurements. As far as implementing this kind of recording as a BMI, I would think there would be many difficulties.
One outstanding question regarding spikes is whether they have significant non-dissipative components. Amoing other things, this would seemingly bear significantly on how much energy they require and carry. Recent studies have attempted to determine exactlyhow much ATP different kinds of neurons need for spiking, however it seems many of their underlying assumptions are dubious. Digant reports that many of the optical pulses have dissipative components as indicated multiple cycles of decaying oscillation after stimulation. He plans to begins studies using optogenetic stimulation to eliminate any artifact introduced by patch clamp.
One good way to get handle on what is going on in spiking cells is to observe what happens when pulses collide. In other words, do they annihilate due to relaxing ion channels like theory predicts, or can they pass through each other? Previous research has found that spikes are naturally propagated in opposite directions down axons, and furthermore that in some cases they can pass right through each other unaffected. Other work has also shown that the speed, amplitude and shape of the spike normally depends on which way it is going. The most recent studies reported here for collisions in Chara cells found that electrically recorded spikes mostly do annihilate upon collision.
The authors suggest that from an acoustic point of view, annihilation can be a result of nonlinear material properties of the entire excitable medium. Because there have been some discrepancies between the phase and directions of cell expansion in different studies with respect to the time course of the electrical spike, optical recordings of collision would probably be informative. We should note that in axons, different protein and lipid compartments can carry different forms of excitation. For example, while ion channels are typically associated with the electrical spike, soliton-like wave phenomena can propagate in bare membranes. In the early days, the original Hodgkin-Huxley papers suggested that membrane dipoles themselves might be responsible for action potentials.
Furthermore, the actin cytoskeleton can also propagate excitation (although pulses generally for longer times as in muscle contraction), and also the tubulin cytoskeleton seems to support excitation and oscillation. As mentioned, the myelin likely also contributes, possibly even through other physical processes like propagating phase changes in lipid components. One thing we might keep in mind for in vivo measurements (particularly for bundled nerves) is that different fascicles may form their own optical sandwich which can be used for the reference optical path length as done for Digand's in vitro work.
A most neglected, but perhaps most important source of excitation in cells or axons may be the mitochondria. In heart cells, for example, the so-called 'mitoflash' response, coordinated by up to 8000 mitochondria per cell, precisely maintains the ATP 'setpoint' across a workload that changes by tenfold. This mitoflash excitation is itself composed of several different components; so-called 'redox sparks', calcium, NADPH, protons, and other molecules have all been recorded, not to mention recent studies showing the interiors of actively respiring mitochondrialcan exceed 50 degrees C. Although controversial, superoxide anion, sometimes associated with direct control of aging and lifespan, has also been presumed to be detected by different mitoflash probes.
Because mitochondria are concentrated at axon internodes it is quite possible that they make significant contribution the the saltatory conduction of spikes in myelinated axons. Considering that the membrane potential in mitochondria is at least double that of the cell itself, and it comes in many small and mobile packages per neuron, this may not be too surprising. Excitability of the whole cell would then be controlled by the dispersion or aggregation of mitochondria into various formations, perhaps akin to how skin color is controlled by strategic mobilization of melanosomes. More locally, mitoflash has been shown to control size and morphology in dendritic spines, leading to wanton speculation regarding memory.
For the BMIs many desire to someday be practical, not only a theory of spikes will be essential, but I'd suggest also the ability to detect, create, or destroy them by the same physical processes that naturally support them.
More information: Subrata Batabyal et al, Label-free optical detection of action potential in mammalian neurons, Biomedical Optics Express (2017). DOI: 10.1364/BOE.8.003700
Abstract
We describe an optical technique for label-free detection of the action potential in cultured mammalian neurons. Induced morphological changes due to action potential propagation in neurons are optically interrogated with a phase sensitive interferometric technique. Optical recordings composed of signal pulses mirror the electrical spike train activity of individual neurons in a network. The optical pulses are transient nanoscale oscillatory changes in the optical path length of varying peak magnitude and temporal width. Exogenous application of glutamate to cortical neuronal cultures produced coincident increase in the electrical and optical activity; both were blocked by application of a Na-channel blocker, Tetrodotoxin. The observed transient change in optical path length in a single optical pulse is primarily due to physical fluctuations of the neuronal cell membrane mediated by a yet unknown electromechanical transduction phenomenon. Our analysis suggests a traveling surface wave in the neuronal cell membrane is responsible for the measured optical signal pulses.
Journal information: Biomedical Optics Express
© 2017 Phys.org