Determining the parameters for transmission electron microscopy
While the phones in our pockets may be perfect for taking photos of our pets, taking good images of catalysts and other materials is far more complex, especially when you bring in scanning transmission electron microscopy (STEM). The STEM imaging method is a way to observe catalysts while they're working, or under catalytic conditions. The challenge is that background scattering from reaction gases, chemical reactions that produce gases, involved lowers the image quality, obscuring vital details about the structure and chemical composition. Dr. Yuanyuan Zhu and Dr. Nigel D. Browning, Pacific Northwest National Laboratory, demonstrated how to effectively image catalysts within the STEM method.
Catalysts are vital to reactions involved in everything from the plastic casing around your phone to the fuel in your car. Creating faster, more efficient catalysts to reduce costs and wastes requires clear and detailed observations of catalysts when reaction gases are introduced to STEM imaging. The new method provides a roadmap for scientists to reduce background diffraction from reaction gases.
Transmission electron microscopy is a projection technique in which a beam of electrons is transmitted through a sample and forms an image. The resulting image can then be focused onto an imaging device and analyzed.
Scanning transmission electron microscopy, referred to as STEM, scans the electron beam across the sample, rather than the beam remaining static. The image shows the electron scattering at each point, providing the mass of each atom. The higher mass of an atom, the brighter it appears in the image. This is known as the Z contrast. Using Z contrast, scientists can determine which individual atoms are reacting in a catalyst.
"The idea is that you can actually see how a catalyst would function by viewing it on the atomic scale," says Browning, catalysis scientist.
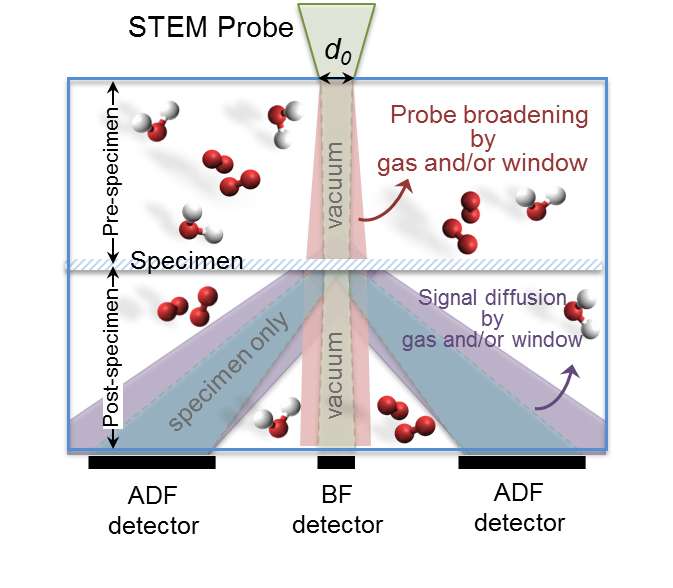
The issue is that when reaction gases are added to the catalyst, the newly introduced gas distorts the image, obscuring the reactions that are occurring. Currently, there is little information on how to properly adjust the reaction gas pressure in the imaging chamber to produce clear and detailed observations.
Zhu and Browning created an in situenvironment in the imaging chamber, meaning that the materials are located within the chamber. They then pumped different reaction gases into the chamber at different pressures to measure the amount of background scattering that occurred in each image. The result was a detailed blueprint of how to subtract the diffraction of the gases to create clear and detailed images that are ready for interpretation.
"This is a method that can be very widely applied to catalysis observations," says Browning. "We've gone through a detailed characterization of how to do that so that anyone else who wants to do these types of experiments can do the same thing and get very detailed [images]."
The next step for Zhu and Browning is to investigate the gas pressure diffraction on different catalysts.
More information: Yuanyuan Zhu et al. The Role of Gas in Determining Image Quality and Resolution During In Situ Scanning Transmission Electron Microscopy Experiments, ChemCatChem (2017). DOI: 10.1002/cctc.201700474
Provided by Pacific Northwest National Laboratory