Control of material crystallization by agitation
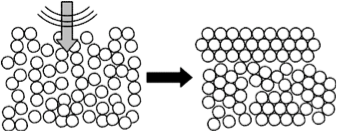
The transition of unstructured amorphous materials into structured crystalline materials is generally induced by heating materials above their transition temperature. Crystalline materials are important in technology like devices, so alternative ways to control their formation has attracted much interest from materials scientists. Researchers have found that crystallization can be facilitated at a lower temperature than the traditional transition temperature if an amorphous material is agitated at a certain frequency. Thus, agitation represents a possible alternative to temperature for controlling the crystallization of materials.
A team at Osaka University decided to clarify the relationship between the agitation and crystallization of amorphous solids. To aid their investigation, they used a colloidal (small particle) system to model atomic materials because the larger particle size and relaxation time of colloids, compared with those of atoms, facilitate their measurement.
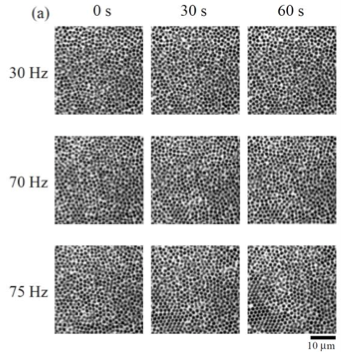
"We prepared colloidal glasses from silica spheres in solution and then oscillated them at different frequencies," explains first author Nobutomo Nakamura. "We then observed the resulting structure by confocal laser scanning microscopy."
The group identified a specific frequency at which crystallization of their system was accelerated. They determined the degree of crystallization in the system agitated at different frequencies by measuring its local bond orientational order parameter. The value of this parameter increased considerably, indicating a higher degree of crystallization, only when the system was agitated at a frequency of around 75 Hz.
"Our results indicated there is a specific vibrational mode that facilitates crystallization of the colloid," says Nakamura.
The researchers then confirmed that the frequency at which crystallization occurs changed depending on the interaction between particles in the system. They added a polymer to the system to alter the interaction force between particles, which caused the crystallization frequency to increase. The team were able to explain their findings by relating the crystallization frequency with the time scale of the vibrational motion of the particles. They proposed that agitation of the system at a frequency that matched the motion of particles forming crystalline structure aided their collective movement and thus accelerated crystallization.
These findings reveal that it may be possible to control the crystallization of amorphous systems by agitation at a specific frequency rather than heating above their transition temperature. This may allow the formation of crystalline materials at a lower temperature, which will be useful in device manufacturing.
More information: Nobutomo Nakamura et al, Accelerated crystallization of colloidal glass by mechanical oscillation, Scientific Reports (2017). DOI: 10.1038/s41598-017-01484-y
Journal information: Scientific Reports
Provided by Osaka University