Making molecules that twinkle
Researchers at Singapore's Agency for Science, Technology and Research (A*STAR) have harnessed the power of carbon dioxide to make two symmetrical star-shaped molecules in a single step. These molecules could be used to build complex, functional polymeric materials useful for personal care, coatings and drug delivery.
Carbon dioxide is a cheap and accessible feedstock that makes it an attractive source of potentially useful molecules. "But transforming carbon dioxide is not typically easy," explains lead researcher Luo He-Kuan from A*STAR's Institute of Materials Research and Engineering.
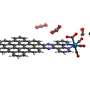
His team has developed a simple route to use carbon dioxide to make functional multi-carbonate compounds that can be used as building blocks for more complicated materials. They created symmetrical benzene rings with three or six identical arms comprising carbonate groups terminated by carbon–carbon triple bonds, or 'alkynes'. "We can integrate the carbon dioxide into the molecule without the need for high temperatures or high pressure," says Luo.
The star-shaped molecules were made in a single step. The team introduced carbon dioxide from dry ice to an alcohol with an alkyne end group and benzene rings decorated with either three or six alkyl bromide groups. "At the beginning, however, only some of the branches reacted so we could not get the desired compound," Luo explains.
The team fine-tuned the process and found the reactions worked most efficiently at room temperature; with the carbon dioxide at atmospheric pressure; and with the addition of a promoter, tetrabutylammonium bromide, and the base, potassium carbonate. "We tried many times and after a few months, we finally got [the bromide groups in] all six branches to react [with the alcohol]," he says.
Adding the promoter to the mix doubled the product produced. "It is likely that the tetrabutylammonium cation enhances the rate of carbon dioxide incorporation by stabilizing the carbonate anion," says Luo.
The reaction time is also vital. "We needed to be patient and let the reaction run to completion to ensure that all the branches reacted." The synthesis of the three-armed and six-armed star-shaped molecule took two and four days respectively.
The alkynes on the end of each arm in these molecules should theoretically be able to react with a host of different molecules to produce a range of complex or functional materials. "We are currently trying to use the six-armed, branched molecule to build more functional star-shaped molecules, which may find applications in personal care, coatings and drug delivery," says Luo.