Study looks to ice for fabricating useful porous materials
Discovering a way to harness ice recrystallization could enable fabrication of highly efficient materials for a range of products, including porous electrodes for batteries and transparent conducting films used to manufacture touch screens and wearable electronics.
A team of researchers from the University of Nebraska-Lincoln and the Chinese Academy of Sciences published findings on the dynamics and manipulation of ice recrystallization in the May 2 issue of Nature Communications.
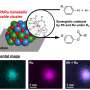
Ice recrystallization is a ubiquitous process in nature. It involves growing large ice crystals at the expense of small ones, leading to an increase in the average crystal size and a decrease in the total number of crystals.
An experimental research group at the Chinese institution has collaborated closely with Xiao Cheng Zeng, Chancellor's University Professor of Chemistry, and Nebraska materials researchers who investigate the properties of water and ice from a computational perspective.
The Chinese group is now using recrystallized ice as a template for synthesizing two- and three-dimensional materials with different pore sizes. Together with their Nebraska colleagues, the team has learned that ions, which are electrically charged molecules, can be used to fabricate new two- and three-dimensional structures on a wide range of other host materials. These technologically important host materials are suitable for organic electronics, catalysis and bioengineering.
"The pore size of two-dimensional and three-dimensional porous materials produced with our method can be easily adjusted, which is critical for practical applications," said project leader Jianjun Wang, a professor in the Institute of Chemistry at the Chinese Academy of Sciences.
"The experimental-theoretical team allows us to work out the problem beautifully because whenever we predict something, they can test it," Zeng said. "Then they can feed back some of the new experimental data, allowing us to reconsider our modeling approach."
Wang's ion-specific recrystallization research stems from his group's cell cryopreservation project. A key reason for cell death during cryopreservation is because large ice crystals grow at the expense of small ones during recrystallization.
During an experiment, one of Wang's students uncovered a striking effect by chance. Adding sodium chloride or phosphate buffer saline produced a profound but previously unexplored effect on the size of recrystallized ice.
In further experiments, Wang's team rapidly froze pure water and three saline solutions, then allowed them to cool at higher temperatures. They found that ions of sodium fluorine produced the smallest ice crystals. Sodium bromine produced larger crystals. Those with sodium iodine produced the largest crystals, which outsized even those produced from pure water.
The Nebraska team conducted molecular dynamics simulations at the Holland Computing Center and the Nebraska Cluster for Computational Chemistry to better understand how fluorine, iodine and bromine ions affect ice recrystallization.
"What we find is that fluorine doesn't get trapped inside the ice, whereas iodine allows that to happen, and to some extent bromine also allows that to happen," Zeng said. "You can use ions to control the ice."
The researchers found that they could tune ice-grain size from approximately 27 microns – roughly half the size of a human hair – to 277 microns.
More information: Shuwang Wu et al. Ion-specific ice recrystallization provides a facile approach for the fabrication of porous materials, Nature Communications (2017). DOI: 10.1038/ncomms15154
Journal information: Nature Communications
Provided by University of Nebraska-Lincoln