March 29, 2017 report
Continuous breathing metal-organic framework with guest selectivity
(Phys.org)—Researchers from the University of Sheffield report a new continuous-breathing metal-organic framework (MOF), SHF-61, that has two different solvent-specific forms, a narrow-pore structure that is the result of DMF or H2O desolvation and a wide-pore structure that is the result of CHCl3 desolvation. The wide-pore form showed uptake of N2, CO2, and CH4 with selectivity for CO2. They were also able to conduct single-crystal structure analysis of their MOF during breathing motions. Their work appears in Nature Chemistry.
"Being modular in construction allows MOFs to be tailored for a wide variety of applications that exploit their molecular-scale porosity. Highly flexible MOFs remain uncommon, but offer the possibility of developing guest-responsive materials. Identifying new flexible MOFs may open many doors for applications, particularly in selective entrapment and release, separation and sensing of molecules," explains Lee Brammer, who is Professor of Inorganic and Solid State Chemistry at the University of Sheffield.
"The flexible behavior of SHF-61 is quite complicated, but what helped in this case is that it proved feasible to study the structural changes in some detail by single-crystal X-ray diffraction."
Breathing MOFs, are metal-organic frameworks whose structure reversibly changes upon some kind of external stimulus. Very few MOFs have been reported to display breathing behavior and of the known MOFs, most undergo some kind of structural change due to a crystal phase transition. This structural change leads to a difference in pore size, which, in turn, allows for the reversible adsorption and desorption of guests. Because these MOFs undergo a phase change, their adsorption profiles (i.e., adsorption isotherms) look like stair steps.
What is not common among breathing MOFs is continuous rather than a stair-step adsorption profile. Continuous breathing MOFs, such as MIL-88, have proven difficult to isolate and study. This paper reports single-crystal and powder XRD studies of continuous-breathing MOF SHF-61.
SHF-61, or (Me2NH2)[In(ABDC)2], where ABDC is 2-aminobenzene-1,4-dicarboxylate, has an In(III) metal coordinated to carboxylates that serve as hinges for the continuous breathing mechanism. The authors point out that the hinge is from rotation of the ABDC ligands around the O—-O of the carboxylates. This is accompanied by changes in the coordination geometry around In(III). The combination of the two motions enables the continuous breathing.
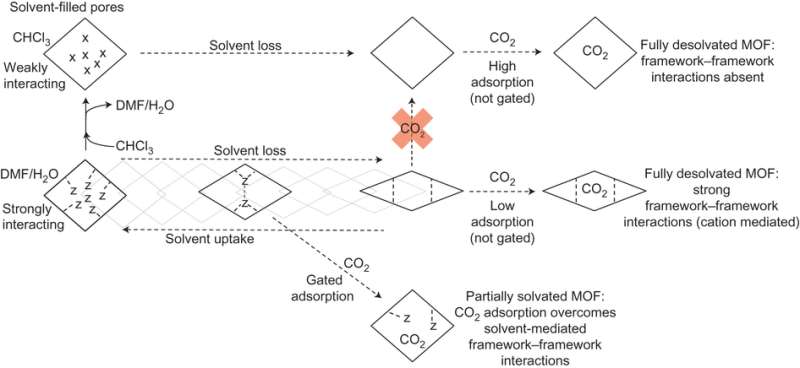
Specifically, In(III) is chelated to four ABDC ligands providing a flattened tetrahedral geometry around the metal center. The resulting anionic framework has diamond-shaped pores, which contain dimethylammonium cations that balance the charge. The pore size is largely dependent on the solvent. Carrington et al. isolated solvated forms of the MOF, SHF-61-DMF and SHF-61-CHCl3, and demonstrated how removal of each solvent affects pore size, and therefore guest uptake, differently. Removal of the more strongly interacting DMF leads to narrowing of the pores whereas removal of the more weakly interacting CHCl3 leaves the pores fully open.
After heating SHF-61-CHCl3 to remove solvent, it exhibited type I adsorption isotherm behavior for N2 and CO2. This was as expected from previous studies. What was new for this study, though, was that CH4 also followed type I adsorption isotherm, but it took much longer for adsorption to occur. This kinetic difference allows for selective adsorption, which has implications for practical uses such as catalysis and separation techniques.
Typically studies with MOFs are either all or nothing in the sense that the adsorption measurements are taken after complete desolvation of the MOF to determine total guest uptake. SHF-61 was also studied for gas adsorption as a partially desolvated MOF, which is the first of this kind of study. The partially desolvated SHF-61-DMF showed a stair-step isotherm instead of type I adsorption isotherm typical of an approximately fixed pore size. The mechanism at work here was identified by in situ powder x-ray diffraction and has to do with a sudden opening of the pores at a particular CO2 pressure threshold.
Finally, while cation-framework interactions are difficult to study, crystallographic studies show that guest-framework and cation-framework interactions control the breathing mechanism, particularly whether the guest is able to overcome cation-framework interactions. This explains the stair-step adsorption pattern for the partially desolvated MOF. While adsorption of CO2 is at first slow, once the pressure of CO2 is high enough to overcome cation-framework interactions, then the pores open allowing for more CO2 to adsorb.
This research demonstrates a unique continuous breathing MOF whose properties have allowed for unprecedented studies in the SHF-61's mechanism and guest selectivity. This research has implications for molecular sensing for gas separation. Because the authors were able to gain new insights into continuous breathing MOFs, future research may include developing other continuous breathing MOFs.
More information: Elliot J. Carrington et al. Solvent-switchable continuous-breathing behaviour in a diamondoid metal–organic framework and its influence on CO2 versus CH4 selectivity, Nature Chemistry (2017). DOI: 10.1038/nchem.2747
Abstract
Understanding the behaviour of flexible metal–organic frameworks (MOFs)—porous crystalline materials that undergo a structural change upon exposure to an external stimulus—underpins their design as responsive materials for specific applications, such as gas separation, molecular sensing, catalysis and drug delivery. Reversible transformations of a MOF between open- and closed-pore forms—a behaviour known as 'breathing'—typically occur through well-defined crystallographic transitions. By contrast, continuous breathing is rare, and detailed characterization has remained very limited. Here we report a continuous-breathing mechanism that was studied by single-crystal diffraction in a MOF with a diamondoid network, (Me2NH2)[In(ABDC)2] (ABDC, 2-aminobenzene-1,4-dicarboxylate). Desolvation of the MOF in two different solvents leads to two polymorphic activated forms with very different pore openings, markedly different gas-adsorption capacities and different CO2 versus CH4 selectivities. Partial desolvation introduces a gating pressure associated with CO2 adsorption, which shows that the framework can also undergo a combination of stepped and continuous breathing.
Journal information: Nature Chemistry
© 2017 Phys.org