Learning how to fine-tune nanofabrication
Daniel Packwood, Junior Associate Professor at Kyoto University's Institute for Integrated Cell-Material Sciences (iCeMS), is improving methods for constructing tiny "nanomaterials" using a "bottom-up" approach called "molecular self-assembly". Using this method, molecules are chosen according to their ability to spontaneously interact and combine to form shapes with specific functions. In the future, this method may be used to produce tiny wires with diameters 1/100,000th that of a piece of hair, or tiny electrical circuits that can fit on the tip of a needle.
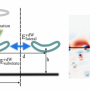
Molecular self-assembly is a spontaneous process that cannot be controlled directly by laboratory equipment, so it must be controlled indirectly. This is done by carefully choosing the direction of the intermolecular interactions, known as "chemical control", and carefully choosing the temperature at which these interactions happen, known as "entropic control".
Researchers know that when entropic control is very weak, for example, molecules are under chemical control and assemble in the direction of the free sites available for molecule-to-molecule interaction. On the other hand, self-assembly does not occur when entropic control is much stronger than the chemical control, and the molecules remain randomly dispersed.
Until now, it's not been possible for researchers to guess what kinds of structures will result from molecular self-assembly when entropic control is neither weak nor strong compared to chemical control.
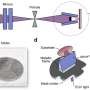
Packwood teamed up with colleagues in Japan and the U.S. to develop a computational method that allows them to simulate molecular self-assembly on metal surfaces while separating the effects of chemical and entropic controls.
This new computational method makes use of artificial intelligence to simulate how molecules behave when placed on a metal surface. Specifically, a "machine learning" technique is used to analyse a database of intermolecular interactions. This machine learning technique builds a model that encodes the information contained in the database, and in turn this model can predict the outcome of the molecular self-assembly process with high accuracy.
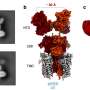
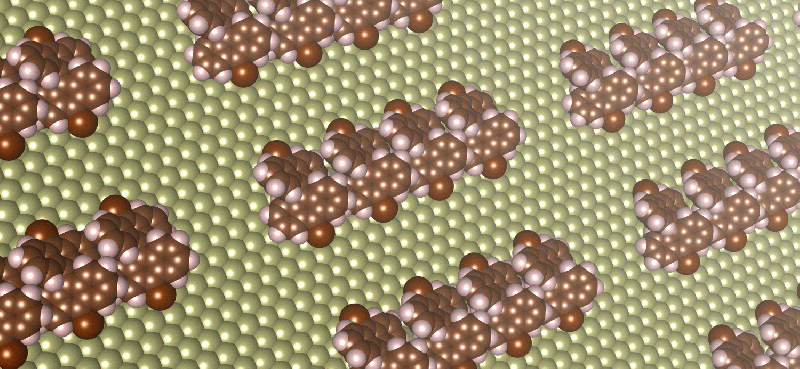
The team used this method to study the self-assembly of three different hydrocarbon molecules, the structures of which vary in the strength of the direction of their intermolecular interactions. In other words, they varied the strength of chemical control by changing the molecule under study.
While stronger chemical control caused molecules to assemble into chain-shaped structures, the effects of stronger entropic controls were found to be more counterintuitive. For example, they found that strengthening entropic control could transform large, disordered structures into several small, ordered, chain-shaped structures. They also showed that the formation of disordered structures results from weak chemical control rather than strong entropic control.
These predictions, which were verified by comparisons with high-resolution microscopic images of real molecules on metal surfaces, may lead to controlled, large-scale fabrication of tiny electrical wires and other nanomaterials for future devices. Devices made from nanomaterials would be significantly smaller and cheaper than existing electronics, and would have very long battery lives due to low energy consumption.
"By continued development of our code and theory, we expect to obtain increasingly detailed rules for controlling molecular self-assembly and aiding the bottom-up nanomaterials fabrication process," the researchers conclude in their study published in the journal Nature Communications.
More information: Daniel M. Packwood et al, Chemical and entropic control on the molecular self-assembly process, Nature Communications (2017). DOI: 10.1038/ncomms14463
Journal information: Nature Communications
Provided by Kyoto University