A cheaper way to make a WHO-designated essential medicine
A fungal form of meningitis leads to more than 600,000 deaths in Africa every year and is responsible for 20 percent of HIV/AIDS-related deaths globally, according to the U.S. Centers for Disease Control and Prevention. An existing medicine could help curb these numbers, but its cost has been a barrier to access in some places. Now, scientists report in the ACS journal Organic Process Research & Development a more affordable way to make the drug.
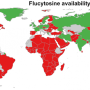
The antifungal flucytosine has been available to patients in the U.S. for decades. In 2011, the World Health Organization (WHO) recommended that patients with Cryptococcal meningitis, an infection of particular concern to people with HIV/AIDS, take flucytosine in combination with amphotericin B as a first line of defense. Flucytosine is now on WHO's Core List of Essential Medicines. However, the drug is not registered for use in many African countries, according to the non-profit Doctors without Borders, and where it is available, many patients can't afford it. Currently, making the drug requires a multiple-step process that involves fluorination, chlorination, amination and hydrolysis from uracil. To help slash flucytosine's price tag and improve its availability, Graham Sandford and colleagues at Durham University in the U.K. wanted to come up with a simpler, lower cost way to make the drug.
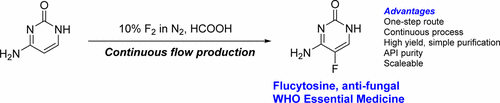
The researchers developed a one-step technique to make flucytosine out of readily available, naturally occurring cytosine. Their process involved simultaneously pumping inexpensive fluorine gas and a solution of cytosine in formic acid through a steel tube. This fluorinated all of the starting cytosine, and the researchers were able to isolate high yields of the resulting flucytosine by recrystallization. The researchers say the method should be simple to scale up for manufacturing and could help lower the drug's cost. The one-step method has been successfully developed to pilot-scale by industrial collaborators Sanofi-Aventis and La Maison Européenne des Procédés Innovants in France.
More information: Antal Harsanyi et al. One-Step Continuous Flow Synthesis of Antifungal WHO Essential Medicine Flucytosine Using Fluorine, Organic Process Research & Development (2017). DOI: 10.1021/acs.oprd.6b00420
Abstract
In Africa around 625 000 mortalities per annum (20% of HIV/AIDS related deaths) are due to the affects of the Cryptococcal meningitis (CM) fungal infection. Recently, the World Health Organisation (WHO) and the Infectious Disease Society of America (IDSA) recommended that the first line treatment for CM is a combination of amphotericin B and flucytosine, both now WHO Essential Medicines. However, flucytosine is not even registered for use in any African nation due, in part, to its relatively high cost of manufacture and lack of generic manufacturers. Currently, flucytosine is manufactured by an expensive four-step manufacturing process. Here we report a one-step continuous flow process involving the reaction of inexpensive cytosine with fluorine gas using stainless steel tubular laboratory and pilot-scale silicon carbide reactor devices which is readily scaleable to a manufacturing process with a low initial capital expenditure.
Journal information: Organic Process Research & Development
Provided by American Chemical Society