February 14, 2017 report
Researchers use new approach to create triangulene molecule
(Phys.org)—A team of researchers with IBM Research in Switzerland and the University of Warwick in the U.K. has successfully created a triangulene molecule by manipulating a precursor molecule physically using a scanning probe microscope tip. In their paper published in the journal Nature Nanotechnology, the team describes their approach and what they have learned about the molecule's properties thus far. Manuel Melle-Franco with the University of Aveiro in Portugal offers a News & Views piece on the work done by the team in the same journal issue.
A triangulene molecule, as its name suggests, is a hydrocarbon molecule that is shaped like a triangle—it is also flat because it is just one atom thick—chemists have been trying in vain for years to synthesize such molecules because of their expected unique properties, but have failed due to the instability of unpaired electrons. In this new effort, the researchers took a new approach, using a scanning probe microscope tip to nudge pieces into place and then to tear away the parts that were not needed.
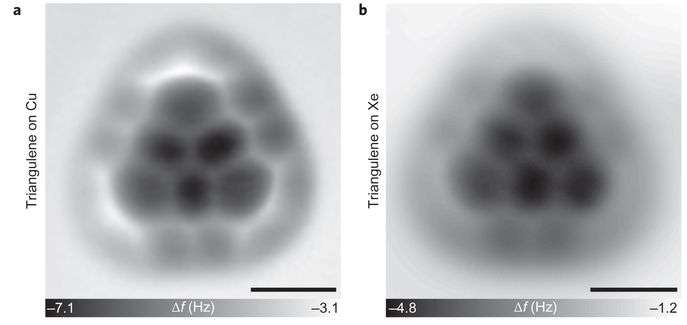
To create the molecule, the researches began with a dihydrotriangulene molecule because it did not have the reactive unpaired electrons—they used it as a precursor, laying it on a base (they tried xenon, copper and salt) and then probing it with the electron microscope tip to get the molecules to align in the desired way. Next, they fired an electron beam at the molecule two times to remove the hydrogen, leaving behind a triangulene. The team then created an image of the molecule they had created using the same microscope, which showed its unique triangular shape. They also found they were able to keep the molecule stable as long as they kept it in a vacuum at low temperatures. In testing the molecule, they found that its two unpaired electrons had aligned spins, which was expected. That property was one of the reasons that chemists have been trying to synthesize the molecule—it is believed it could prove very useful in various electronic devices and might even have applications in a quantum computer.
The research team plans to continue their work with creating triangulene molecules to learn more about their properties and possible uses. They also hope to find out why their technique worked when a copper bases was used—they thought the two materials would react.
More information: Niko Pavliček et al. Synthesis and characterization of triangulene, Nature Nanotechnology (2017). DOI: 10.1038/nnano.2016.305
Abstract
Triangulene, the smallest triplet-ground-state polybenzenoid (also known as Clar's hydrocarbon), has been an enigmatic molecule ever since its existence was first hypothesized. Despite containing an even number of carbons (22, in six fused benzene rings), it is not possible to draw Kekulé-style resonant structures for the whole molecule: any attempt results in two unpaired valence electrons. Synthesis and characterization of unsubstituted triangulene has not been achieved because of its extreme reactivity, although the addition of substituents has allowed the stabilization and synthesis of the triangulene core and verification of the triplet ground state via electron paramagnetic resonance measurements. Here we show the on-surface generation of unsubstituted triangulene that consists of six fused benzene rings. The tip of a combined scanning tunnelling and atomic force microscope (STM/AFM) was used to dehydrogenate precursor molecules. STM measurements in combination with density functional theory (DFT) calculations confirmed that triangulene keeps its free-molecule properties on the surface, whereas AFM measurements resolved its planar, threefold symmetric molecular structure. The unique topology of such non-Kekulé hydrocarbons results in open-shell π-conjugated graphene fragments6 that give rise to high-spin ground states, potentially useful in organic spintronic devices. Our generation method renders manifold experiments possible to investigate triangulene and related open-shell fragments at the single-molecule level.
Journal information: Nature Nanotechnology
© 2017 Phys.org