January 11, 2017 feature
Scientists create first 2-D electride
(Phys.org)—Researchers have brought electrides into the nanoregime by synthesizing the first 2D electride material. Electrides are ionic compounds, which are made of negative and positive ions. But in electrides, the negative "ions" are simply electrons, with no nucleus. The electrons are very close to each other and very loosely bound, causing them to act as an electron gas. This electron gas gives electrides certain electrical properties, such as a high electrical mobility and rapid electrical transport, that are very attractive for electronics applications.
The researchers, led by Scott C. Warren, an assistant professor of applied physical sciences and chemistry at the University of North Carolina at Chapel Hill, have published a paper on the demonstration of the 2D electride in a recent issue of the Journal of the American Chemical Society.
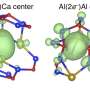
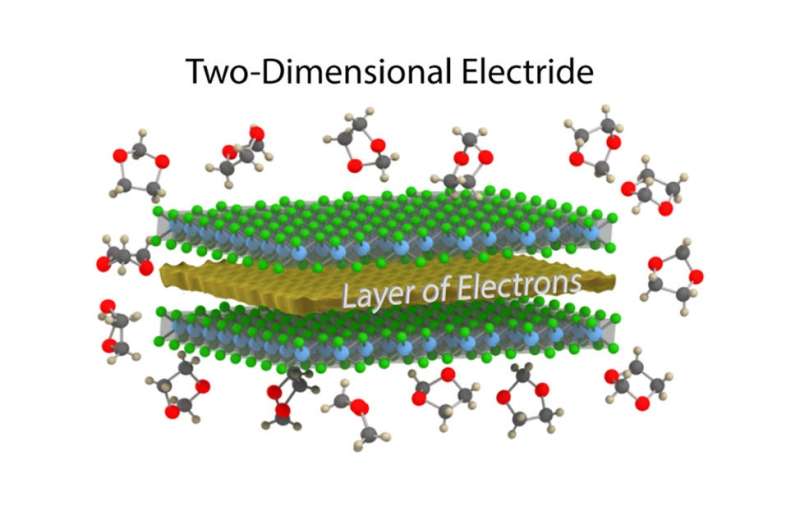
"Layered electrides have very exciting electronic properties—for example, a conductivity much greater than that of graphene," Warren told Phys.org. "In the crystal structure of a layered electride, a cloud of electrons is spread out into a flat two-angstrom-thick plane between slabs of atoms. The electrons can conduct through that flat cloud with few interactions with nearby atoms, allowing them to move very quickly."
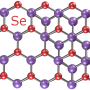
In their study, the researchers showed that the defining features of electrides—in particular, the electron gas and its properties—are preserved when a layered electride called dicalcium nitride (Ca2N) is synthesized in two-dimensional, single-layer form. The work marks the first synthesis of a 2D electride.
"We have isolated a few layers of the crystal, perhaps as thin as a nanometer to several nanometers," Warren said. "Because of its thinness, this material is called a 2D material, like graphene. An electride as a 2D material had been predicted to be stable in vacuum and to retain its exciting electronic qualities by theoretical calculations, but the material is very reactive and it was an open question whether 2D Ca2N could be made in a lab setting. We showed that in the right chemical environment, the material is stable for long periods of time without compromising its exciting electronic properties."
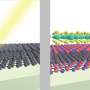
As the researchers explained, separating the multilayered electride into its individual layers was challenging since electrides have strong electrostatic interactions that hold their layers together. Electrides also have a high chemical reactivity that further complicates matters, preventing the use of the "Scotch-tape method" for exfoliation since electrides decompose when coming into contact with certain adhesives.
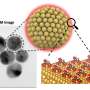
Instead, the researchers used liquid exfoliation, which uses chemical reactions to produce large numbers of nanosheets suspended in solution. After testing 30 solvents, the researchers found one solvent in which the Ca2N nanosheets remain in a stable suspension for at least a month.
Tests showed that the 2D electride nanosheets have high electrical conductivity comparable to aluminum metal, high transparency (a 10-nm-thick film transmits 97% of light), and—due to the 2D form—the highest surface area for any electride reported to date. By combining the high surface area of 2D materials with the unusual electrical properties of electrides, the researchers expect that the 2D electride will lead to many more discoveries in the future. Potential applications include transparent conductors, battery electrodes, electron emitters, and catalysts for chemical synthesis.
"The potential application that excites us the most is in advanced batteries, which is the focus of our current collaboration with the Honda Research Institute," Warren said. "There are other exciting potential applications too, for example as transparent conductive films. From an academic perspective, this work opens up synthetic routes to study 2D electrides experimentally and to test potential applications that we haven't even considered yet."
In the future, the researchers plan to further explore the potential applications of electrides and address the practical challenges in realizing them.
"We have a lot to learn about electrides as 2D materials," Warren said. "For example, how can we coat or functionalize the surface to make electrides stable in air?"
More information: Daniel Druffel et al. "Experimental Demonstration of an Electride as a 2D Material." Journal of the American Chemical Society. DOI: 10.1021/jacs.6b10114
Journal information: Journal of the American Chemical Society
© 2017 Phys.org