Carbon-free energy from solar water splitting
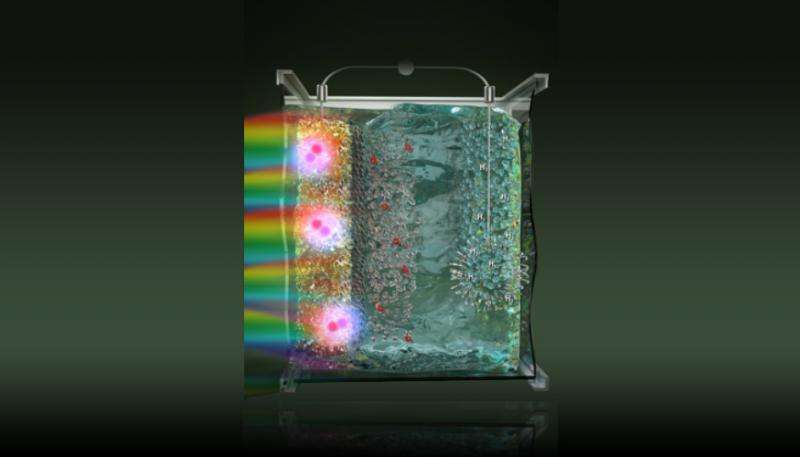
A Lawrence Livermore National Laboratory (LLNL) scientist and collaborators are fine tuning the mechanisms to generate hydrogen from water and sunlight.
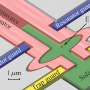
Hydrogen production offers a promising approach for producing scalable and sustainable carbon-free energy. The key to a successful solar-to-fuel technology is the design of efficient, long-lasting and low-cost photoelectrochemical cells (PECs), which are responsible for absorbing sunlight and driving water-splitting reactions.
LLNL's Lawrence Fellow Anh Pham, Assistant Professor Yuan Ping from the University of California at Santa Cruz and Professor Giulia Galli from the University of Chicago and Argonne National Laboratory (formerly an LLNL scientist) reviewed the use of first-principles methods to understand the interfaces between photoabsorbers, electrolytes and catalysts in PECs.
The key to building an efficient PEC relies on the availability of abundant semiconducting photoelectrode materials that are responsible for absorbing sunlight and driving water-splitting reactions.
"Despite steady efforts and some breakthroughs, no single material has yet been found that simultaneously satisfies the efficiency and stability required for the commercialization of PEC hydrogen production technology," Pham said.
The research appears in the Jan. 9 edition of the journal Nature Materials .
The team shows that with growing complexity of PEC architectures, understanding the properties of the interfaces between its components is key to predict novel, better performing materials and eventually to optimize the device performance.
In this study, the team discussed open challenges in describing PEC interfaces using first-principles techniques, focusing on the interplay between their structural and electronic properties. The scientists also reviewed first-principles techniques relevant for the study of solid-liquid interfaces, the structural and electronic properties of photoelectrode-water and photoelectrode-catalyst water interfaces and open theoretical challenges in the simulation of PEC interfaces.
More information: Tuan Anh Pham et al. Modelling heterogeneous interfaces for solar water splitting, Nature Materials (2017). DOI: 10.1038/nmat4803
Journal information: Nature Materials
Provided by Lawrence Livermore National Laboratory