Researchers turn back the clock on human embryonic stem cells
Johns Hopkins scientists report success in using a cocktail of cell-signaling chemicals to further wind back the biological clock of human embryonic stem cells (ESCs), giving the cells the same flexibility researchers have prized in mice ESCs.
The investigators say the ability to reset the stem cells' developmental clock to an earlier stage offers new opportunities to successfully coax human stem cells into making any kind of cell on demand for use as transplants and in genetic disease modeling. Eventually, they may be used to create chimeric animals from which human organs could be harvested.
Reporting on their work in the Nov. 29 Development, the researchers wrote that their so-called 3i cocktail, named for its three chemical inhibitors, produced stem cells with all the same features of classic mouse ESCs: they are easy to grow, manipulate and steer to differentiate into a variety of cell types—without the genetic instability that resulted from previous efforts to transform human stem cells.
"These cells are exactly what we've been hoping for ever since the first human ESCs were derived," says Elias Zambidis, M.D., Ph.D., associate professor of oncology at the Johns Hopkins University School of Medicine and member of the Johns Hopkins Kimmel Cancer Center and Institute for Cell Engineering.
When the first human ESCs were isolated in 1998, researchers working with these cells quickly noticed differences between them and those isolated nearly two decades earlier from mice. Mouse ESCs easily thrived in petri dishes, were able to generate nearly every cell or tissue type, could be genetically manipulated without much effort, and could make chimeras (organisms containing cells with at least two different sets of DNA).
However, researchers found it far more difficult to coax conventional human ESCs into similarly performing these tasks. The human cells were more difficult to keep alive in laboratory cultures, could make only a limited selection of tissue types with far more work, and inserting or removing genes took substantially more effort. Researchers also had similar difficulties with other human stem cells derived from mature adult cells through a process called induced pluripotency.
"For many years, people just kind of shrugged their shoulders and said, 'Humans and mice are different species. You can't expect their cells to behave the same,'" says Zambidis.
Then, in 2007, researchers discovered a new type of primitive mouse stem cell known as an epiblast stem cell, derived from cells just a couple of days older than classic mouse ESCs. Rather than the facile features of their conventional mouse ESC counterparts, these epiblast stem cells behaved like the less malleable conventional human ESCs already in use. Suddenly, says Zambidis, many researchers began to suspect that human ESCs were more akin to these less pliable mouse epiblast stem cells and not to the more versatile mouse ESCs, and that an authentic human ESC with more useful features had yet to be discovered.
Getting human ESCs to revert to the classical mouse ESC state, however, has proven difficult, Zambidis says. In 2015, several research groups published scientific findings suggesting that dosing conventional human ESCs with certain chemical mixtures could create this "ground state" similar to mouse ESCs, but subsequent research showed that these cells may have unstable genetic properties that may be related to the chemicals used to create them.
In a new effort to create more malleable human ESCs, Zambidis and his colleagues dosed conventional ESCs with novel combinations of chemicals known to regulate well-studied developmental signaling pathways.
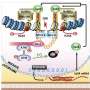
Eventually, he says, they hit on a mixture of just three compounds that inhibit signaling pathways known to be pivotal when early cells mature into defined cell types. Two of the compounds in the 3i cocktail, inhibitors of the WNT and MEK/ERK signaling pathways, were previously used to help mouse embryonic stem cells maintain a primitive state. The third chemical that Zambidis and his colleagues added to the mixture is an anti-cancer agent called a tankyrase inhibitor.
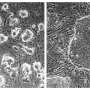
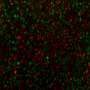
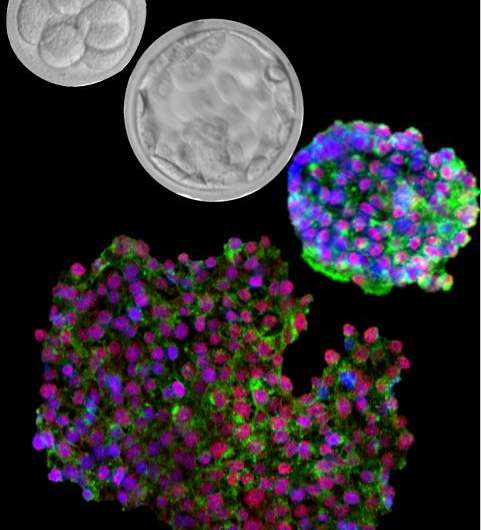
Zambidis and his team were able to "reset" a broad range of more than 25 human stem cell lines by using this new cocktail of three chemical inhibitors. They showed that these reset human ESCs expressed genes and proteins common only in the more malleable mouse ESCs, as well as in human preimplantation embryos, but not in conventional human ESCs. They also found these newly reverted human ESCs did not have the abnormal changes in their DNA that other methods induced. The new human ESCs differentiated into transplantable vascular and neural cell types at double or triple the frequencies of conventional human ESCs.
Zambidis says he and his colleagues are now using this new class of human ESCs to develop blood, vascular and retinal tissues that are more suitable for transplantation than those previously derived from conventional human ESCs. Zambidis says this new class of human ESCs is more amenable to correction of genes known to be associated with diseases that include sickle cell disease and Parkinson's disease. The new ESCs could also create chimeras in which human organs can grow in animals and provide a potentially unlimited source of transplantable organs, he says. His team is now studying the cellular mechanisms behind this new 3i cocktail to better understand how it rewinds the clock in these cells.
More information: Ludovic Zimmerlin et al. Tankyrase inhibition promotes a stable human naïve pluripotent state with improved functionality, Development (2016). DOI: 10.1242/dev.138982
Journal information: Development
Provided by Johns Hopkins University School of Medicine