Exotic property of salty solutions discovered
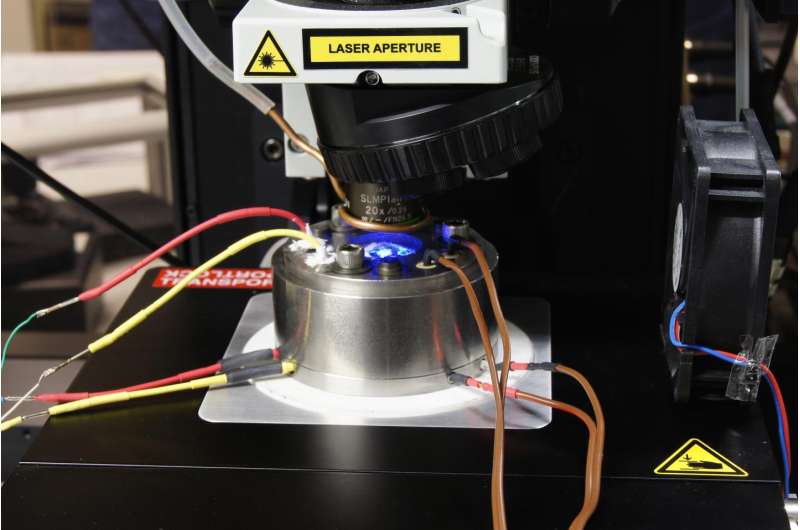
Water and aqueous solutions can behave strangely under pressure. Experiments carried out at the GFZ German Research Centre for Geosciences using Raman spectroscopy and a diamond anvil cell showed that magnesium sulfate dissolved in water was separated less than expected in magnesium and sulfate ions above a pressure of 0.2 Gigapascal, which equals 2,000 times the normal air pressure. Moreover, ion pairing even increased with pressure above about 0.5 Gigapascal.
This is the opposite of the predicted trend that salt solutions become more dissociated with increasing pressure. However, the previously unknown anomaly was only observed at comparably low temperatures. Already at 50 °C, the solutions behaved as expected. "That's why this effect does not occur in the Earth's interior", says Christian Schmidt of the GFZ, "as the pressure in our oceans is not high enough even in the deep-sea trenches, and the temperature is too high in the Earth's crust and mantle."
Still, the anomaly is relevant for studies on cold planetary bodies with deep oceans. Christian Schmidt and Craig Manning of the University of California in Los Angeles (UCLA) published their findings in the journal Geochemical Perspectives Letters.
Their results may help in studies of the oceans that are probably hidden under thick icy shells in Pluto and in the moons Ganymede, Callisto, and Titan. It is very likely that magnesium sulfate is the major or among the most abundant solutes in these oceans, because it is generated by weathering of magnesium silicates in ocean floors. If more ion pairs form, magnesium silicate weathering is enhanced. "This means that the oceans in these icy worlds are probably saltier than currently thought", says Christian Schmidt. As the ion concentration determines the electrical conductivity of aqueous solutions, the finding will help to better interpret magnetometric data obtained by spacecrafts.
The experiments were carried out at the GFZ's section "Chemistry and Physics of Earth Materials". The scientists explain the observed anomaly with a change in the dynamic structure of water that is generated by hydrogen bonds between water molecules.
More information: C. Schmidt et al, Pressure-induced ion pairing in MgSO4 solutions: Implications for the oceans of icy worlds, Geochemical Perspectives Letters (2017). DOI: 10.7185/geochemlet.1707
Provided by Helmholtz Association of German Research Centres