Scientists use neutrons to understand the secrets of extremophile bacteria
Microorganisms represent the most numerous life on earth and the understanding of how they behave is integral to our own survival and well-being. Microbial life has an amazing flexibility for adapting to extreme environments—niches that are extraordinarily hot or cold, acidic or basic, salty as in the Dead Sea or at high pressure in great ocean depths—that would be detrimental to complex organisms. These organisms are known as extremophiles.
Among the extremophiles, bacteria, isolated from salt marshes or marine environments include a variety of interesting species of high biotechnological potential such as the recently discovered Halomonas titanicae in the hull of the sunken RMS Titanic ship. It has been estimated that the action of this rust-producing Halomonas may bring about the total deterioration of the Titanic by 2030; in the same way it has been identified as a potential danger to oil rigs and other man-made objects in the deep sea. But the rusting property could also be harnessed in bioremediation or waste management, for example to accelerate the decomposition of shipwrecks littering the ocean floor.
A range of experiments conducted at the world's leading neutron research centre, the Institut Laue-Langevin (ILL), in collaboration with Max Planck Institute of Biochemistry (MPIB), Bitop biotechnology company and the Institut de Biologie Structurale (IBS), concentrated on the interaction of ectoine with water and protein.
Ectoine is a natural compound found in many organisms, including Halomonas. It serves as a protective substance by acting as an osmolyte – a molecule that plays a role in fluid balance and cell volume maintenance and thus helps organisms survive extreme environmental stress. Ectoine is called a compatible solute in the sense that its occurrence within the internal material of the cell does not interfere with cellular biochemistry and metabolism. Halomonas can produce ectoine up to an intracellular concentration of 20% of the cellular dry mass. By this adaptive regulatory process, the microorganism is said to be halotolerant over a broad range of salt concentration, from 0.5 to 25% NaCl (on average, sea water has a salt concentration of 3.5%). Ectoine itself, which displays an indirect stabilising effect on proteins and membranes and a related inhibitory effect on inflammation in mammalian cells, has found broad cosmetic and clinical applications through its hydration, stabilisation and inflammation reducing properties, e.g. for the treatment of allergies, atopic dermatitis or cough and cold symptoms.
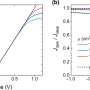
Neutrons, used in combination with advanced deuterium labelling methods, have illustrated how ectoine acts by leaving intact the shell of water on protein and membrane surfaces, which is essential to their biological activity. H2O molecules in liquid water interact with each other through a highly dynamic fluid network of hydrogen bonds (H-bonds) between the O and H atoms of adjacent molecules. The presence of other substances in the water can hamper this organisation. The neutron experiments went on to describe the effects of ectoine on water H-bond dynamics to reveal how ectoine's protective characteristics do not interfere with the cell metabolism. In fact, ectoine, rather than hindering, enhances the remarkable dynamic properties of H-bonds in water―properties that are essential for water's unique solvent capabilities, and vital for the proper organisation, stabilisation and function of proteins, lipids, membranes, RNA and DNA.
Dr Joe Zaccai, Emeritus Scientist of the CNRS at the ILL says: "It is well-known that the search for Life on Mars and elsewhere in the universe, is guided by a search for liquid water. This is because liquid water is essential for all Life. Its remarkable properties are based on dynamic H-bond networks that play vital roles in macromolecular folding and interactions, which in turn determine their biological functions. The results in this study illustrate how the osmolyte behind the halotolerance response in microorganisms induces compensating effects on water H-bonding that respect these essential biological properties. Neutrons provide the ideal tool for investigating structure and dynamics in water and biological molecules–they have a number of unique advantages, including amongst others their high penetrative power with no radiation damage to the sample and their sensitivity to labelling a structure by replacing hydrogen with its isotope deuterium. Each of the instruments used in the study acted like a 'giant microscope' of different magnification for us to 'see' details from the crucial hydrogen-bond formations at the atomic level to the larger protein and membrane structures. Although much spectroscopic and thermodynamic investigations have been done before on ectoine, we are proud that through the use of neutrons this is the first study that has allowed a direct experimental characterisation of ectoine-water-protein and ectoine-water-membrane structures to explain the mode of action of this very interesting and useful molecule."
More information: Giuseppe Zaccai et al. Neutrons describe ectoine effects on water H-bonding and hydration around a soluble protein and a cell membrane, Scientific Reports (2016). DOI: 10.1038/srep31434
Journal information: Scientific Reports
Provided by CNRS