Scientists discover interplay of yin-yang antagonists vital for cell division
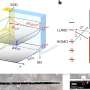
How a cell transitions from the G1 phase of the cell cycle to the S phase – a fundamental process of cell division for all eukaryotic cells on Earth – has been a long-studied question in biology. Now UNC School of Medicine scientists discovered that this boundary is regulated by a pair of large enzyme complexes that attack each other in turn to form a molecular switch, which eventually allows the cell cycle to enter into S phase.
The discovery, published in today in Cell Reports, raises the possibility that future drugs could target this enzyme interaction to help stop the uncontrolled division of cancerous cells. Above all, though, it illuminates the workings of one of the most important processes in biology.
"Our work describes a key piece of the puzzle for how this fundamental process works," said Michael J. Emanuele, PhD, study senior author, assistant professor in department of pharmacology, and member of the UNC Lineberger Comprehensive Cancer Center. "We did not focus on suppressing cancer cell division in this study, but given what we've found we think it's definitely worth investigating further."
The crossing from G1 to S phase is effectively a point of no return for a cell. Once past that threshold it must divide or die trying. Whereas in G1 phase the cell merely ramps up its production of proteins and other key molecules so that the two future daughter cells will have enough to live. Later, in S phase, the cell becomes committed to the division process by duplicating and then separating all of its chromosomes.
Standing guard at this boundary between phases is a molecular machine known as APC/C, which comprises more than a dozen separate proteins including an enzyme component.
Other scientists found that APC/C teams up with a protein called Cdh1 to grab a bunch of other proteins in the cell and tag them (with enzymes) for disposal by a roving protein-cruncher known as the proteasome. Cells commonly use tagging-for-disposal complexes like APC/C – which are known as E3 ubiquitin ligases – to regulate the amounts of specific proteins. The proteins that APC/C suppresses include many that would facilitate the cell's entry into S-phase. Thus, while APC/C remains active, the cell cannot move into S-phase.
"Shutting off APC/C is essential for cells to get across the G1/S border," Emanuele said. "We've known that for a really long time, and we've also known that this requirement exists in the cells of all organisms from yeast to humans. But just how APC/C is shut off to permit this border crossing has not been clear."
The journey to their discovery began when Emanuele's lab found that APC/C and Cdh1 target a protein called cyclin F. The finding was interesting because cyclin F does what Cdh1 does; it serves as a target-recognition device for another disposal-tagging E3 ubiquitin ligase, in this case one called SCF. Thus, one big ubiquitin ligase, APC/C-Cdh1, effectively shuts down SCF-cyclin F.
Emanuele and his team soon found hints that SCF-cyclin F returns the favor.
"When we looked at cells going through the cell cycle, we saw cyclin F levels starting to increase right at the G1/S transition, and at the same time the levels of Cdh1 were decreasing. The timing was perfect," Emanuele said. "Then, when we put the protein complexes in cells at the same time, the levels of both went down, as if they were targeting each other."
Further experiments confirmed that while APC/C-Cdh1 targets cyclin F and thus shuts down SCF/cyclin F, the latter reciprocally targets Cdh1 and thus shuts down APC/C-Cdh1. It is that second "flip of the switch" that permits the cell's entry into S-phase.
"To the best of our knowledge nobody else has described a system involving direct antagonism between two E3 ubiquitin ligases," Emanuele said. "It suggests the possibility that similar mutually antagonistic pairs of E3s regulate other oscillating systems in biology."
Precisely how APC/C establishes dominance to prevent S phase, and how SCF/cyclin F gets the upper hand to permit S phase, isn't yet clear. However, cells do have a variety of systems for the dynamic control of protein activity, such as phosphorylation, which can change a protein's shape and thereby render it active or inactive. Emanuele suspects that phosphorylation or some similar modification may be what triggers the ascendancy of each of these ubiquitin ligases in turn. "That's something we're working on now," he said.
The research illuminates new pathways of investigation into other cell division mysteries, including how cell division goes out of control in cancer.
"Cdh1 is a largely under-appreciated tumor suppressor," Emanuele said. "Although it blocks S phase, Cdh1 isn't mutated in cancers. One of the implications of our data, however, is that Cdh1 is degraded at this critical G1/S juncture. It could be that in some cases, too much Cdh1degradation is all that's needed to promote cancerous growth."
Journal information: Cell Reports
Provided by University of North Carolina Health Care System