Mystery solved? Biologists find a unique version of a filament-forming protein in human cells that insects lack
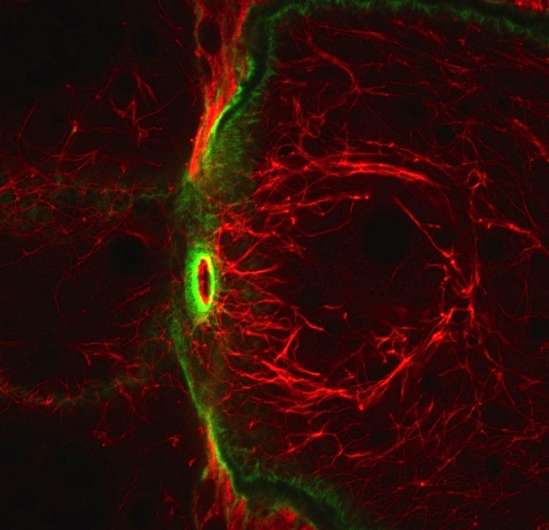
Providing structural support and protection against such conditions as blistering, cataracts and dementia, intermediate filament proteins (IFs) reside in every cell in the human body. In insects, however, IFs are nowhere to be found.
Scientists posit that another kind of protein has taken over key IF functions in insects, but exactly what kind—or even where to start looking—has been a mystery.
New research conducted by UC Santa Barbara biologist Denise Montell and her team of scientists may have solved the conundrum. They discovered a protein in fruit flies that demonstrates IF-like characteristics. An unusual form of the protein tropomyosin was present in every cell type they examined. Their findings appear in the journal Cell Reports.
"Believe it or not, fruit flies and humans have a lot of proteins in common, including conventional tropomyosin," said Montell, the Robert and Patricia Duggan Chair in Mathematical, Life, and Physical Sciences in UCSB's Department of Molecular, Cellular, and Developmental Biology.
"Evolution started with tropomyosin and morphed it into a brand new protein with very different characteristics simply by adding a few bits to each end," she explained. "Like IFs—but unlike normal tropomyosin—this new protein makes filaments that are 'intermediate' in size. This means they are larger than F-actin filaments and smaller than microtubules, two other important structural filaments in cells."
This new type of filament-forming protein may substitute for some intermediate filaments in flies.
Normal tropomyosin forms a ropelike structure—a so-called coiled coil—from one end to the other. This skinny tropomyosin rope normally wraps around F-actin filaments. Coiled in only one small region, this newly discovered version doesn't look or act like a normal tropomyosin.
"We knew what it wasn't, but we wanted to know what it was," said Montell, whose team studies many different aspects of biology in fruit flies, including how stem cells are maintained and how cells move from one place to another. "The bits that had been added to the ends reminded us of IF proteins, so we decided to test whether it might be the long-sought intermediate filament substitute protein in flies."
To better understand the function of this unique molecule, the researchers examined the uncoiled regions at each end of the protein. A computer program predicted those areas to be intrinsically disordered low-complexity domains, meaning they lack one stable structure.
"While this configuration—coiled coil with intrinsically disordered domains at each end—resembled IF proteins generally, the novel tropomyosin differed from IFs in the details," Montell explained. "Still, we wondered whether this atypical protein might have filament-making capabilities or the ability to perform IF-like functions."
Masato Kato, a colleague at the University of Texas Southwestern Medical Center in Dallas, produced the protein in a test tube. He then varied the conditions—like pH and salt concentration—that work for different kinds of IF proteins. He found conditions in which the atypical tropomyosin from flies formed 13- to 16-nanometer-wide filaments that had never been described before.
"We found all kinds of similarities in biochemical behavior between this protein and IF proteins despite the differences in sequence," Montell said. "Because our protein seems to have some properties in common with IFs, it may be the answer to the long-standing question of how flies survive without actual IF proteins. But like any exciting discovery, it also raises many more questions."
More information: An Atypical Tropomyosin in Drosophila with Intermediate Filament-like Properties , Cell Reports, www.cell.com/cell-reports/full … 2211-1247(16)30808-7 , DOI: dx.doi.org/10.1016/j.celrep.2016.06.054
Journal information: Cell Reports
Provided by University of California - Santa Barbara